8
Auditory Prostheses
From the Lab to the
Clinic and Back Again
- 8.1 Hearing Aid Devices Past and Present
- 8.2 The Basic Layout of Cochlear Implants
- 8.3 Place Coding with Cochlear Implants
- 8.4 Speech Processing Strategies Used in Cochlear Implants
- 8.5 Pitch and Music Perception Through Cochlear Implants
- 8.6 Spatial Hearing with Cochlear Implants
- 8.6 Brain Plasticity and Cochlear Implantation
Hearing research is
one of the great scientific success stories of the last 100 years. Not only have
so many interesting discoveries been made about the nature of sound and the
workings of the ear and the auditory brain, but these discoveries have also
informed many immensely useful technological developments in entertainment and
telecommunications as well as for clinical applications designed to help
patients with hearing impairment.
Having valiantly worked your way
through the previous chapters of the book, you will have come to appreciate
that hearing is a rather intricate and subtle phenomenon. And one sad fact
about hearing is that, sooner or later, it will start to go wrong in each and
every one of us. The workings of the middle and inner ears are so delicate and
fragile that they are easily damaged by disease or noise trauma or other
injuries, and even those who look after their ears carefully cannot reasonably
expect them to stay in perfect working order for over 80 years or longer. The
consequences of hearing impairment can be tragic. No longer able to follow
conversations with ease, hearing impaired individuals can all too easily become
deprived of precious social interaction, stimulating conversation, and the joy
of listening to music. Also, repairing the auditory system when it goes wrong
is not a trivial undertaking, and many early attempts at restoring lost auditory
function yielded disappointing results. But recent advances have led to
technologies capable of transforming the lives of hundreds of thousands of deaf
and hearing impaired individuals. Improved hearing aid and cochlear implant
designs now enable many previously profoundly deaf people to pick up the phone
and call a friend.
And just as basic science has been
immensely helpful in informing design choices for such devices, the successes,
limitations, or failures of various designs are, in turn, scientifically
interesting, as they help to confirm or disprove our notions of how the
auditory system operates. We end this book with a brief chapter on hearing aids
and cochlear implants in particular. This chapter is not intended to provide a
systematic or comprehensive guide to available devices, or to the procedures
for selecting or fitting them. Specialized audiology texts are available for
that purpose. The aim of this chapter is rather to present a selection of
materials chosen to illustrate how the fundamental science that we introduced
in the previous chapters relates to practical and clinical applications.
8.1 Hearing Aid Devices Past and Present
As we mentioned in
chapter 2, by far the most common cause of hearing loss is damage to cochlear
hair cells, and in particular to outer hair cells whose purpose seems to be to
provide a mechanical amplification of incoming sounds. Now, if problems stem
from damage to the ear’s mechanical amplifier, it would make sense to try to
remedy the situation by providing alternative means of amplification. Hearing
loss can also result from pathologies of the middle ear, such as otosclerosis, a disease in which slow, progressive bone
growth on the middle ear ossicles reduces the
efficiency with which airborne sounds are transmitted to the inner ear. In such
cases of conductive hearing loss, amplification of the incoming sound can also be
beneficial.
The simplest and oldest hearing
aid devices sought to amplify the sound that enters the ear canal by purely
mechanical means. So-called ear trumpets were relatively widely used in the
1800s. They funneled collected sound waves down a narrowing tube to the ear
canal. In addition to providing amplification by collecting sound over a large
area, they had the advantage of fairly directional acoustic properties, making
it possible to collect sound mostly from the direction of the sound source of
interest.
Ear trumpets do, of course, have
many drawbacks. Not only are they fairly bulky, awkward, and technologically
rather limited, many users would also be concerned about the “cosmetic side
effects.” Already in the 1800s, many users of hearing aids were concerned that
these highly conspicuous devices might not exactly project a very youthful and
dynamic image. Developing hearing aids that could be hidden from view therefore
has a long history. King John VI of

Figure 8.1
A
chair with in-built ear trumpet, which belonged to King John VI of
The design of King John VI’s chair is certainly ingenious, but not very practical.
Requiring anyone who wishes to speak with you to kneel and address you through
the jaws of your carved lion might be fun for an hour or so, but few
psychologically well-balanced individuals would choose to hold the majority of
their conversations in that manner. It is also uncertain whether the hearing
aid chair worked all that well for King John VI. His reign was beset by
intrigue, both his sons rebelled against him, and he ultimately died from
arsenic poisoning. Thus, it would seem that the lion’s jaws failed to pick up
on many important pieces of court gossip.
Modern hearing aids are thankfully
altogether more portable, and they now seek to overcome their cosmetic
shortcomings not by intricate carving, but through miniaturization, so that
they can be largely or entirely concealed behind the pinna
or in the ear canal. And those are not the only technical advances that have
made modern devices much more useful. A key issue in hearing aid design is the
need to match and adapt the artificial amplification provided by the device to
the specific needs and deficits of the user. This is difficult to do with
simple, passive devices such as ear trumpets. In cases of conductive hearing
loss, all frequencies tend to be affected more or less equally, and simply
boosting all incoming sounds can be helpful. But in the countless patients with
sensorineural hearing loss due to outer hair cell
damage, different frequency ranges tend to be affected to varying extents. It
is often the case that sensorineural hearing loss
affects mostly high frequencies. If the patient is supplied with a device that
amplifies all frequencies indiscriminately, then such a device would most
likely overstimulate the patient’s still sensitive
low-frequency hearing before it amplifies the higher frequencies enough to
bring any benefit. The effect of such a device would be to turn barely
comprehensible speech into unpleasantly loud booming noises. It would not make
speech clearer.
You may also remember from section
2.3 that the amplification provided by the outer hair cells is highly nonlinear
and “compressive,” that is, the healthy cochlea amplifies very quiet sounds
much more than moderately loud ones, which gives the healthy ear a very wide
“dynamic range,” allowing it to process sounds over an enormous amplitude range.
If this nonlinear biological amplification is replaced by an artificial device
that provides simple linear amplification, users often find environmental
sounds transition rather rapidly from barely audible to uncomfortably loud.
Adjusting such devices to provide a comfortable level of loudness can be a
constant struggle. To address this, modern electronic hearing aids designed for
patients with sensorineural hearing loss offer nonlinear
compressive amplification and “dynamic gain control.”
Thus, in recent years, hearing aid
technology has become impressively sophisticated, and may incorporate highly
directional microphones, as well as digital signal processing algorithms that transpose
frequency bands, allowing information in the high-frequency channels in which
the patient has a deficit to be presented to the still intact low-frequency
part of the cochlea. And for patients who cannot receive the suitably
amplified, filtered, and transposed sound through the ear canal, perhaps
because of chronic or recurrent infections, there are even bone-anchored
devices that deliver the sound as mechanical vibration of a titanium plate
embedded in the skull, or directly vibrate the middle ear ossicles
by means of a small transducer system implanted directly in the middle ear.
With such a wide variety of
technologies available, modern hearing aid devices can often bring great
benefit to patients, but only if they are carefully chosen and adjusted to fit
each patient’s particular needs. Otherwise they tend to end up in a drawer,
gathering dust. In fact, this still seems to be the depressingly common fate of
many badly fitted hearing aids. It has been estimated (Kulkarni
& Hartley, 2008) that, of the 2 million hearing aids owned by hearing
impaired individuals in the UK in 2008, as many as 750,000, more than one
third, are not being used on a regular basis, presumably because they fail to
meet the patient’s needs. Also, a further 4 million hearing impaired
individuals in the
To work at their best, hearing
aids should squeeze as much useful acoustic information as possible into the
reduced frequency and dynamic range that remains available to the patient. As
we shall see in the following sections, the challenges for cochlear implant
technology are similar, but tougher, as cochlear implants have so far been used
predominantly in patients with severe or profound hearing loss (thresholds
above 75 dB SPL) over almost the entire frequency range, so very little normal
functional hearing is left to work with.
8.2 The Basic Layout of Cochlear Implants
Cochlear implants are
provided to the many patients who are severely deaf due to extensive damage to
their hair cells. Severe hearing loss caused by middle ear disease can often be
remedied surgically. Damaged tympanic membranes can be repaired with skin
grafts, and calcified ossicles can be trimmed or
replaced. But at present there is no method for regenerating or repairing
damaged or lost sensory hair cells in the mammalian ear. And when hair cells
are lost, the auditory nerve fibers that normally connect to them are
themselves at risk and may start to degenerate. It seems that auditory nerve
fibers need to be in contact with hair cells to stay in the best of health. But
while this anterograde degeneration of denervated auditory nerve fibers is well documented, and
can even lead to cell death and shrinkage in the cochlear nuclei, it is a very
slow process and rarely leads to a complete degeneration of the auditory
afferents. Also, a patient’s hearing loss is often attributable to outer hair
cell damage. Without the amplification provided by these cells, the remaining
inner hair cells are incapable of providing sensitive hearing, but they
nevertheless survive and can exercise their beneficial trophic
influences on the many type I auditory nerve fibers that contact them.
Consequently, even after many years of profound deafness, most hearing impaired
patients still retain many thousand auditory nerve fibers, waiting for auditory
input. Cochlear implants are, in essence, simple arrays of wires that stimulate
these nerve fibers directly with pulses of electrical current.
Of course, the electrical
stimulation of the auditory nerve needs to be as targeted and specific as one
can make it. Other cranial nerves, such as the facial nerve, run close to the
auditory branch of the vestibulocochlear nerve, and
it would be unfortunate if electrical stimulus pulses delivered down the
electrodes, rather than evoking auditory sensations, merely caused the
patient’s face to twitch. To achieve highly targeted stimulation with an
extracellular electrode, it is necessary to bring the electrode contacts into
close proximity to the targeted auditory nerves. And to deliver lasting
benefits to the patient, they need to stay there, for many years. At present,
practically all cochlear implant devices in clinical use achieve this by
inserting the electrode contacts into the canals of the cochlea.
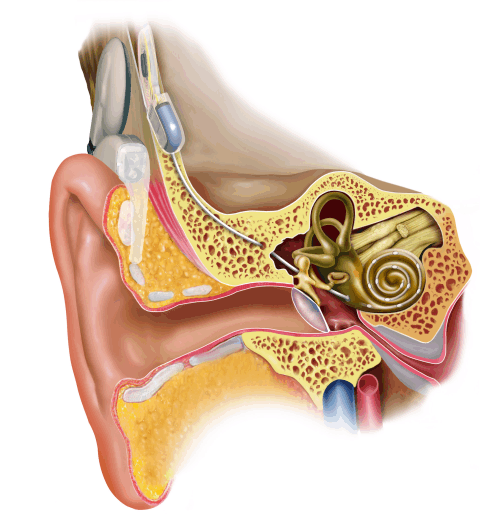
Figure 8.2
A
modern cochlear implant. Image kindly provided by
Figure 8.2 shows the layout of a typical
modern cochlear implant. The intracochlear electrode
is made of a plastic sheath fitted with electrode contacts. It is threaded into
the cochlea, either through the round window or through a small hole drilled
into the bony shell of the cochlea just next to the round window. The electrode
receives its electrical signals from a receiver device, which is implanted
under the scalp, on the surface of the skull, somewhat above and behind the
outer ear. This subcutaneous device in turn receives its signals and its
electrical energy via an induction coil from a headpiece radio transmitter.
Small, strong magnets fitted into the subcutaneous receiver and the transmitter
coil hold the latter in place on the patient’s scalp. The transmitter coil in
turn is connected via a short cable to a “speech processor,” which is usually
fitted behind the external ear. This speech processor collects sounds from the
environment through a microphone and encodes them into appropriate electrical
signals to send to the subcutaneous receiver. It also supplies the whole
circuitry with electrical power from a battery. We will have a lot more to say
about the signal processing that occurs in the speech processor in just a
moment, but first let us look in a bit more detail at how the intracochlear electrode is meant to interface with the
structures of the inner ear.
When implanting the device, the
surgeon threads the electrode through a small opening at or near the round
window, up along the scala tympani, so as to place
the electrode contacts as close as possible to the modiolus
(the center of the cochlear helix). Getting the electrode to “hug the modiolus” is thought to have two advantages: First, it
reduces the risk that the electrode might accidentally scratch the stria vascularis that runs along
the opposite external wall of the cochlear coil, and could bleed easily and
cause unnecessary trauma. Second, it is advantageous to position the electrode
contacts very close to the AN fibers, whose cell bodies, the spiral ganglion
cells, live in a cavity inside the modiolus known as
Rosenthal’s canal.
Inserting an electrode into the
cochlea is a delicate business. For example, it is thought to be important to
avoid pushing the electrode tip through the basilar membrane and into the scala media or scala vestibuli, as that could damage AN
fiber axons running into the organ of Corti. And, it
is also thought that electrode contacts that are pushed through into the scala media or scala vestibuli are much less efficient at stimulating AN fibers than those that sit on the modiolar
wall of the scala tympani. You may recall that the
normal human cochlea helix winds through two and a half turns. Threading an
electrode array from the round window through two and a half turns all the way
up to the cochlear apex is not possible at present. Electrode insertions that cover
the first, basal-most one to one and a quarter turns are usually as much as can
reasonably be achieved. Thus, after even a highly successful CI operation, the
electrode array will not cover the whole of the cochlea’s tonotopic
range, as there will be no contacts placed along much of the apical, low-frequency
end.
Through its electrode contacts,
the cochlear implant then aims to trigger patterns of activity in the auditory
nerve fibers, which resemble, as much as possible, the activity that would be
set up by the synaptic inputs from the hair cells if the organ of Corti on the basilar membrane was functional. What such normal
patterns of activity should look like we have discussed in some detail in
chapter 2. You may recall that, in addition to the tonotopic
place code for sound frequency and the spike rate coding for sound intensity, a
great deal of information about a sound’s temporal structure is conveyed
through phase locked temporal discharge patterns of auditory nerve fibers. This
spike pattern information encodes the sound’s amplitude envelope, its
periodicity, and even the submillisecond timing of
features that we rely on to extract interaural time differences for spatial
hearing. Sadly, the intracochlear electrode array
cannot hope to reproduce the full richness of information that is encoded by a
healthy organ of Corti. There are quite serious
limitations of what is achievable with current technology, and many compromises
must be made. Let us first consider the place coding issue.
8.3 Place Coding with Cochlear Implants
If the electrodes can
only cover the first of the two and a half turns of the cochlea, then you might
expect that less than half of the normal tonotopic
range is covered by the electrode array. Indeed, if you look back at figure 2.3,
which illustrated the tonotopy of the normal human basilar membrane, you will
see that the first, basal-most turn of the human cochlea covers the
high-frequency end, from about 20 to 1.2 kHz or so. The parts of
the basilar membrane that are most sensitive to
frequencies lower than that are beyond the reach of current cochlea implant
designs. This high-frequency bias could be problematic. You may recall that the
formants of human speech, which carry much of the phonetic information, all
evolve at relatively low frequencies, often as low as just a few hundred hertz.
Poor coverage of the low-frequency
end is an important limitation for cochlear implants, but it is not as big a
problem as it may seem at first glance, for two reasons. First, Rosenthal’s
canal, the home of the cell bodies of the auditory nerve fibers, runs alongside
the basilar membrane for only about three quarters of its length, and does not
extend far into the basilar membrane’s most apical turn. The spiral ganglion
cells that connect to the low-frequency apical end of the basilar membrane
cover the last little stretch with axons that fan out over the last turn,
rather than positioning themselves next to the apical points on the basilar
membrane they innervate (Kawano, Seldon, & Clark,
1996). Consequently, there is a kind of anatomical compression of the tonotopy
of the spiral ganglion relative to that of the organ of Corti,
and an electrode array that runs along the basilar membrane for 40% of its
length from the basal end may nevertheless come into close contact with over
60% of spiral ganglion cells. Second, it seems that our auditory system can
learn to understand speech fairly well even if formant contours are shifted up
in frequency. In fact, implantees who had previous
experience of normal hearing often describe the voices they hear through the
implants as “squeaky” or “Mickey Mouse-like” compared to the voices they used
to experience, but they can nevertheless understand the speech well enough to
hold a telephone conversation.
Trying to get cochlear implants to
cover a wide range of the inner ear’s tonotopy is one issue. Another technical
challenge is getting individual electrode contacts to target auditory nerve
fibers so that small parts of the tonotopic array can
be activated selectively. It turns out that electrical stimuli delivered at one
point along the electrode array tend to spread sideways and may activate not
just one or a few, but many neighboring frequency channels. This limits the
frequency resolution that can be achieved with cochlear implants, and
constitutes another major bottleneck of this technology.
Obviously, the number of separate frequency
channels that a cochlear implant delivers can never be greater than the number
of independent electrode contacts that can be fitted on the implant. The first
ever cochlear implants fitted to human patients in the 1950s had just a single
channel (Djourno & Eyries,
1957), and were intended to provide a lip-reading aid and generate a basic
sound awareness. They were certainly not good enough to allow the implantees to understand speech. At the time of writing,
the number of channels in implants varies with the manufacturer, but is usually
less than twenty five. This is a very small number given that the device is
aimed to replace tonotopically organized input from
over 3,000 separate inner hair cells in the normal human cochlea. You might
therefore think that it would be desirable to increase this number further.
However, simply packing greater
and greater numbers of independent contacts onto the device is not enough. Bear
in mind that the electrode contacts effectively float in the perilymphatic fluid that fills the scala
tympani, and any voltage applied to the contacts will provoke current flows that
may spread out in all directions, and affect auditory nerve fibers further
afield almost as much as those in the immediate vicinity. There is little point
in developing electrodes with countless separate contacts if the “cross talk”
between these contacts is very high and the electrodes cannot deliver
independent information because each electrode stimulates very large, and
largely overlapping, populations of auditory nerve fibers. Somehow the
stimulating effect of each electrode contact must be kept local.
One strategy that aims to achieve
this is the use of so-called bipolar, or sometimes even tripolar,
rather than simple monopolar electrode
configurations. A bipolar configuration, rather than delivering voltage pulses
to each electrode contact independently, delivers voltages pulses of opposite
polarity in pairs of neighboring electrode site. Why would that be
advantageous? An electrode influences neurons in its vicinity by virtue of the
electric field it generates when a charge is applied to it (compare figure
8.3). The field will cause any charged particles in the
vicinity, such as sodium ions in the surrounding tissue, to feel forces of
electrostatic attraction toward or repulsion away from the electrode. These
forces will cause the charges to move, thus setting up electric currents, which
in turn may depolarize the membranes of neurons in the vicinity to the point
where these neurons fire action potentials. The density of the induced currents
will be proportional to the voltage applied, but will fall off with distance
from the electrode according to an inverse square law. Consequently, monopolar electrodes will excite nearby neurons more easily
than neurons that are further away, and while this decrease with distance is
initially dramatic, it does “level off” to an extent at greater distances. This
is illustrated in figure 8.3A.
The little arrows point in the direction of the electric force field, and their length is proportional to the logarithm of
the size of the electric forces available to drive currents at each point.
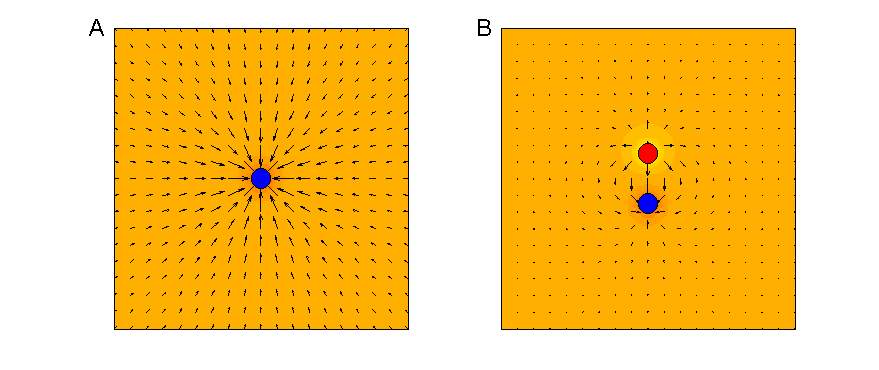
Figure 8.3
Electric fields
created by monopolar (A) and bipolar (B) electrodes.
In a bipolar electrode arrangement,
as illustrated in figure 8.3 B, equal and opposite
charges are present at a pair of electrode contacts in close vicinity. Each of
these electrodes produces its own electrostatic field, which conforms to the
inverse square law, and a nearby charged particle will be attracted to one and
repelled by the other electrode in the pair. However, seen from points a little
further afield, the two electrodes with their opposite charges may seem to lie
“more or less equally far away,” and in “more or less the same direction,” and
the attractive and repulsive forces exercised by the two electrodes will
therefore cancel each other at these more distant points. Over distances that are
large compared to the separation of the electrodes, the field generated by a
bipolar electrode therefore declines much faster than one generated by a monopolar electrode. (Note that the electric field arrows
around the edges of figure 8.3B
are shorter than those in A.) In theory, it should therefore be possible to
keep the action of cochlear implant electrodes more localized when the
electrode contacts are used in pairs to produce bipolar stimulation, rather
than driving each electrode individually.
We say “in theory,” because
experimental evidence suggests that, in practice, the advantage bipolar
electrode arrangements offer tends to be modest. For example, Bierer and Middlebrooks (2002)
examined the activation of cortical neurons achieved in a guinea pig that had
received a scaled-down version of a human cochlear implant. The implant had six
distinct stimulating electrode sites, and brief current pulses were delivered,
either in a monopolar configuration, each site being
activated in isolation, or in a bipolar mode, with pairs of adjacent electrodes
being driven in opposite polarities. Cortical responses to the electrical
stimuli were recorded through an array of sixteen recording electrodes placed
along a 1.5-mm-long stretch of the guinea pig cortex, a stretch that covers the
representation of about two to three octaves of the tonotopic
axis in this cortical field. Figure
8.4 shows a representative example of the data they
obtained.
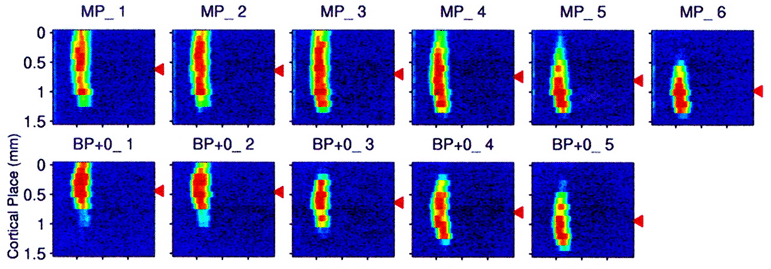
Figure 8.4
Activation
of guinea pig auditory cortex in response to stimulation at five different sites
on a cochlear implant with either monopolar (MP) or
bipolar (BP) electrode configuration.
Adapted from figure 4 of Bierer and Middlebrooks (2002) J Neurophysiol 87:478-492, with permission from The American
Physiological Society..
The gray scale shows the
normalized spike rate observed at the cortical location, shown on the y-axis at
the time following stimulus onset on the x-axis. Cortical neurons responded to
the electrical stimulation of the cochlea with a brief burst of nerve impulses.
And while any one stimulating electrode caused a response over a relatively
wide stretch of cortical tissue, the “center of gravity” of the neural activity
pattern (shown by the black arrow heads) nevertheless shifts systematically as
a function of the site of cortical stimulation. The activation achieved in
bipolar mode is somewhat more focal than that seen
with monopolar stimulation, but the differences are
not dramatic.
Experiments testing the level of
speech comprehension that can be achieved by implanted patients also fail to
show substantial and consistent advantages of bipolar stimulation (
Of course, for electrodes inserted
into the scala tympani, the partition wall between
the scala tympani and Rosenthal’s canal sets absolute
limits on how close the contacts can get to the neurons they are meant to
stimulate, and this in turn limits the number of distinct channels of acoustic
information that can be delivered through a cochlear implant. If electrodes
could be implanted in the modiolus or the auditory
nerve trunk to contact the auditory nerve fibers directly, this might increase
the number of well-separated channels that could be achieved, and possible
designs are being tested in animal experiments (Middlebrooks
& Snyder, 2007). But the surgery involved in implanting these devices is
somewhat riskier, and whether such devices would work well continuously for
decades is at present uncertain. Also, in electrode arrays that target the
auditory nerve directly, working out the tonotopic
order of the implanted electrodes is less straightforward.
With any stimulating electrode,
larger voltages will cause stronger currents, and the radius over which neurons
are excited by the electrode will grow accordingly. The duration of a current
pulse also plays a role, as small currents may be sufficient to depolarize a
neuron’s cell membrane to threshold provided that they are applied for long
enough. To keep the effect of a cochlear implant electrode localized to a small
set of spiral ganglion cells, one would therefore keep the stimulating currents
weak and brief, but that is not always possible because cochlear implants
signal changes in sound level by increasing or decreasing stimulus current, and
implantees perceive larger currents (or longer
current pulses) as “louder” (McKay, 2004; Wilson, 2004). In fact, quite modest
increases in stimulus current typically evoke substantially louder sensations.
In normal hearing, barely audible
sounds would typically be approximately 90 dB weaker than sounds that might be
considered uncomfortably loud. In cochlear implants, in contrast, electrical
stimuli grow from barely audible to uncomfortably loud if the current amplitude
increases by only about 10 dB or so (McKay, 2004; Niparko,
2004). One of the main factors contributing to these differences between
natural and electrical hearing is, of course, that the dynamic range
compression achieved by the nonlinear amplification of sounds through the outer
hair cells in normal hearing, which we discussed in section 2.3, is absent in
direct electrical stimulation. Cochlear implants, just like many digital
hearing aids, must therefore map sound amplitude onto stimulus amplitude in a
highly nonlinear fashion.
Stronger stimulating currents,
which signal louder sounds, will, of course, not just drive nearby nerve fibers
more strongly, but also start to recruit nerve fibers increasingly further
afield. In other words, louder sounds may mean poorer channel separation. To an
extent this is a natural state of affairs, since, as we have seen in section
2.4, louder sounds also produce suprathreshold
activation over larger stretches of the tonotopic
array in natural hearing. However, we have also seen that, in natural hearing,
temporal information provided through phase locking may help disambiguate place-coded
frequency information at high sound levels (compare, for example, sections 2.4
and 4.3). In this manner, phase-locked activity with an underlying 500-Hz
rhythm in a nerve fiber with a characteristic frequency of 800 Hz would be
indicative of a loud 500-Hz tone. As we discuss further below, contemporary
cochlear implants are sadly not capable of setting up similar temporal fine
structure codes.
A lateral spread of activation
with higher stimulus intensities could become particularly problematic if
several electrode channels are active simultaneously. As we have just seen in
discussing bipolar electrodes, cancellation of fields from nearby electrode
channels can be advantageous (even if the benefits are in practice apparently not
very large). Conversely, it has been argued that “vector addition” of fields
generated by neighboring electrodes with the same polarity could be deleterious
(McKay, 2004; Wilson & Dorman, 2009). Consequently, a number of currently
available speech processors for cochlear implants are set up to avoid such
potentially problematic channel interaction by never firing more than one
electrode at a time. You may wonder how that can be done; after all, many
sounds we hear are characterized by their distribution of acoustic energy across
several frequency bands. So how is it possible to convey fairly complex
acoustic spectra, such as the multiple formant peaks of a vowel, without ever firing
more than one channel at a time? Clearly, this involves some trickery and some
compromises, as we shall see in the next sections, where we consider the
encoding of speech, pitch, and sound source location through cochlear implants.
8.4 Speech Processing Strategies Used in Cochlear Implants
You will recall from
chapter 4 that, at least for English and most other Indo-European languages,
semantic meaning in speech is carried mostly by the time-varying pattern of
formant transitions, which are manifest as temporal modulations of between 1
and 7 Hz and spectral modulations of less than 4 cycles/kHz.
Consequently, to make speech comprehensible, neither the spectral nor the temporal
resolutions need to be very high. Given the technical difficulties involved in
delivering a large number of well-separated spectral channels through a
cochlear implant, this is a distinct advantage. In fact, a paper by Bob Shannon
and colleagues (1995) described a nice demonstration that as few as four
suitably chosen frequency channels can be sufficient to achieve good speech
comprehension. This was done by using a signal-processing technique known as
“noise vocoding,” which bears some similarity to the
manner in which speech signals are processed for cochlear implants. Thus, when
clinicians or scientists wish to give normally hearing individuals an idea of
what the world would sound like through a cochlear implant, they usually use
noise vocoded speech for these demonstrations. You
can find examples of noise vocoded sounds on the Web
site that accompanies this book <flag>. Further work by Dorman, Loizou, and Rainey (1997) has extended
The first step in noise vocoding, as well as in speech processing for cochlear
implants, is to pass the recorded sound through a series of bandpass
filters. This process is fundamentally similar to the gamma-tone filtering we
described in sections 1.5 and 2.4 as a method for modeling the function of the
basilar membrane, only speech processors and noise vocoders
tend to use fewer, more broadly tuned, nonoverlapping
filters. Figure 8.5C
illustrates the output of such a filter bank, comprising six bandpass filters, in response to the acoustic waveform shown
in figure 8.5A
and B.
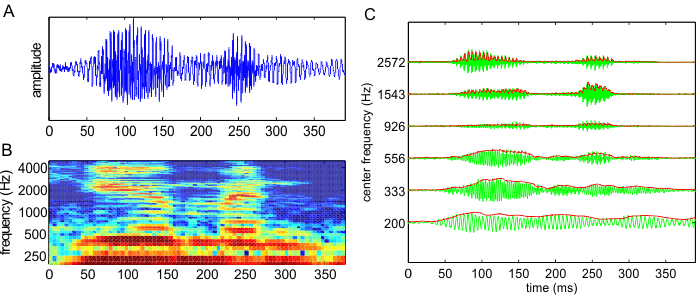
Figure 8.5
(A) Waveform of the
word “human” spoken by a native American speaker. (B)
Spectrogram of the same word. (C) Gray lines: Output of a set of six bandpass filters in response to the same word. The filter
spacing and bandwidth in this example are two-thirds of an octave. The center
frequencies are shown in the y-axis. Black lines: amplitude envelopes of the
filter outputs, as estimated with half-wave rectification and bandpass filtering.
Bandpass filtering similar to that shown
in figure 8.5 is the first step in the
signal processing for all cochlear implant speech processors, but processors
differ in what they then do with the output of the filters. One of the simplest
processing strategies, referred to as the simultaneous
analog signal (SAS) strategy, uses the filter outputs more or less directly
as the signal that is fed to the stimulating electrodes. The filter outputs
(shown by the gray lines in figure 8.5C)
are merely scaled to convert them into alternating currents of an amplitude
range that is appropriate for the particular electrode site they are sent to.
The appropriate amplitude range is usually determined when the devices are
fitted, simply by delivering a range of amplitudes to each site and asking the
patient to indicate when the signal becomes uncomfortably loud. A close cousin
of the SAS strategy, known as compressive
analog (CA), differs from SAS only in the details of the amplitude scaling
step.
Given that speech comprehension
usually requires only modest levels of spectral resolution, SAS and CA speech
processing can support good speech comprehension even though these strategies
make no attempt to counteract potentially problematic channel interactions. But
speech comprehension with SAS declines dramatically if there is much background
noise, particularly if the noise is generated by other people speaking in the
background, and it was thought that more refined strategies that might achieve
a better separation of a larger number of channels might be beneficial. The
first strategy to work toward this goal is known as continuous interleaved sampling (CIS). A CIS device sends a train
of continuous pulses to each of the electrode channels (
Note, by the way, that the pulses
used in CIS, and indeed in all pulsatile stimulation
for cochlear implants, are “biphasic,” meaning that each current pulse is
always followed by a pulse of opposite polarity, so that, averaged over time,
the net charge outflow out of the electrodes is zero. This is done because,
over the long term, net currents flowing from or into the electrodes can have
undesirable electrochemical consequences, and provoke corrosion of the
electrode channels or toxic reactions in the tissue.
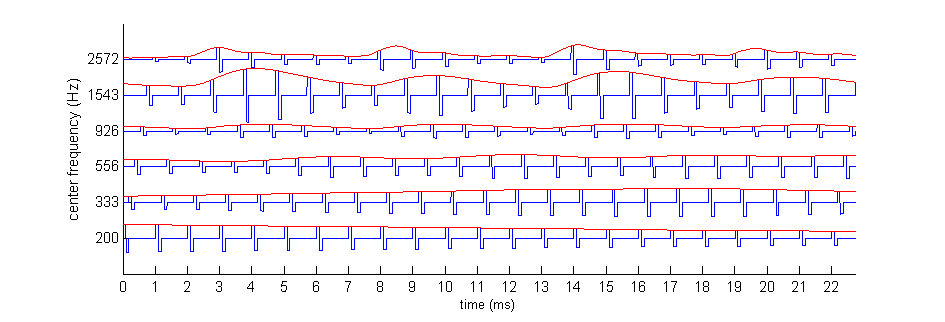
Figure 8.6
The
continuous interleaved sampling (CIS) speech coding strategy. Amplitude envelopes from the
output of a bank of bandpass filters (black dashed
lines; compare figure 8.5C) are used to modulate the
amplitude of regular biphasic pulse trains (shown in gray). The pulses for
different frequency bands are offset in time so that no two pulses are on at
the same time. The modulated pulses are then delivered to the cochlear implant
electrodes.
Because in CIS, no two electrode
channels are ever on simultaneously, there is no possibility of unwanted
interactions of fields from neighboring channels. The basic CIS strategy
delivers pulse trains, like those shown in figure 8.6,
to each of the electrode contacts in the implant. A number of implants now have
more than twenty channels, and the number of available channels is bound to
increase as technology develops. One reaction to the availability of an
increasing number of channels has been the emergence of numerous variants of
the CIS strategy which, curiously, deliberately choose not to use all the
available electrode channels. These strategies, with names like n-of-m, ACE (“advanced
combinatorial encoder”), and SPEAK ( “spectral peak”),
use various forms of “dynamic peak picking” algorithms. Effectively, after
amplitude extraction, the device uses only the amplitude envelopes of a modest
number of frequency bands that happened to be associated with the largest
amplitudes, and switches the low-amplitude channels off. The rationale is to
increase the contrast between the peaks and troughs of the spectral envelopes
of the sound, which could help create, for example, a particularly salient
representation of the formants of a speech signal.
While all of these variants of CIS
still use asynchronous, temporally interleaved pulses to reduce channel
interactions, there are also algorithms being developed that deliberately
synchronize current pulses out of adjacent electrodes in an attempt to create
“virtual channels” by “current steering” (Wilson & Dorman, 2009). At its
simplest, by simultaneously activating two adjacent channels, the developers
are hoping to produce a peak of activity at the point between the two
electrodes. However, the potential to effectively “focus” electrical fields
onto points between the relatively modest number of contacts on a typical electrode
is, of course, limited, and such approaches have so far failed to produce
substantial improvements in speech recognition scores compared to older
techniques. For a more detailed description of the various speech-processing
algorithms in use today, the interested reader may turn to reviews by Wilson
and colleagues (2004; 2009), which also discuss the available data regarding
the relative effectiveness of these various algorithms.
One thing we can conclude from the
apparent proliferation of speech-processing algorithms in widespread clinical
use is that, at present, none of the available algorithms is clearly superior
to any of the others. As
8.5 Pitch and Music Perception Through Cochlear Implants
As we have just seen,
the majority of cochlear implant speech-processing strategies in use today rely
on pulsatile stimulation, where pulses are delivered
at a relatively high rate (1 kHz or more) to each stimulating electrode. The
pulse rate is the same on each electrode, and is constant, independent of the
input sound. The rationale behind this choice of pulse train carriers is to
deliver sufficiently well-resolved spectral detail to allow speech recognition
through a modest array of electrode contacts, which suffer from high levels of
electrical cross-talk. But when you cast your mind back to our discussions of
phase locking in chapter 2, and recall from chapter 3 that phase locking
provides valuable temporal cues to the periodicity, and hence the pitch, of a
complex sound, you may appreciate that the constant-rate current pulse carriers
used in many cochlear implant coding strategies are in some important respects
very unnatural. CIS or similar stimulation strategies provide the auditory
nerve fibers with very little information about the temporal fine structure of
the sound. Phase locking to the fixed-rate pulsatile
carrier itself would transmit no information about the stimulus at all. Some
fibers might conceivably be able to phase lock to some extent to the amplitude
envelopes in the various channels (rounded to the nearest multiple of the
carrier pulse rate), but the amplitude envelopes used in CIS to modulate the
pulse trains are low-pass filtered at a few hundred hertz to avoid a phenomenon
called “aliasing.” Consequently, no temporal cues to the fundamental frequency
of a complex sound above about 300 Hz survive after the sound has been processed
with CIS or a similar strategy. And to infer the fundamental frequency from the
harmonic structure of the sound would, as you may recall, require a very fine
spectral resolution, which even a healthy cochlea may struggle to achieve, and
which is certainly beyond what cochlear implants can deliver at present or in
the foreseeable future. With these facts in mind, you will not be surprised to
learn that the ability of implantees to distinguish
the pitches of different sounds tends to be very poor indeed, with many implantees struggling to discriminate even pitch intervals
as large as half an octave or greater (Sucher & McDermott,
2007).
Against this background, you may
wonder to what extent it even makes sense to speak about pitch perception at
all in the context of electrical hearing with cochlear implants. This is a
question worth considering further, and in its historical context (McKay,
2004). As you may recall from chapter 3, the American National Standards
Institute (1994) defines pitch as “that auditory attribute of sound according
to which sounds can be ordered on a scale from low to high.” As soon as
cochlear implants with multiple electrode sites became available, researchers
started delivering stimulus pulses to either an apical or a basal site, asking
the implantees which electrical stimulus “sounded
higher.” In such experiments, many implantees would
reliably rank more basal stimulation sites as “higher sounding” than apical
sites. These reported percepts were therefore in line with the normal cochlear tonotopic order. However, if, instead of delivering
isolated pulses to various points along the cochlea, one stimulates just a
single point on the cochlea with regular pulse trains and varies the pulse
rates over a range from 50 to 300 Hz, implantees will
also report higher pulse rates as “higher sounding,” even if the place of
stimulation has not changed (McKay, 2004; Moore and Carlyon, 2005; Shannon,
1983).
If you find these observations
hard to reconcile, then you are in good company. Moving the site of stimulation
toward a more basal location is a very different manipulation from increasing
the stimulus pulse rate. How can they both have the same effect, and lead to a
higher sounding percept? One very interesting experiment by Tong and colleagues
(1983) suggests how this conundrum might be solved: Fast pulse rates and apical
stimulation sites may both sound “high,” but the “direction” labeled as “up” is
not the same in both cases. Tong et al. (1983) presented nine different
electrical pulse train stimuli to their implantee
subjects. The stimuli encompassed all combinations of three different pulse
rates and three different cochlear places, as shown in the left of figure
8.7. But instead of presenting the electrical stimuli in
pairs and asking “which one sounds higher,” the stimuli were presented in
triplets, and the subjects were asked “which two of the three stimuli sound
most alike.” By repeating this process many times with many different triplets
of sounds, it is possible to measure the “perceptual distance” or perceived
dissimilarity between any two of the sounds in the stimulus set. Tong and
colleagues then subjected these perceptual distance estimates to a multidimensional
scaling (MDS) analysis.
The details of MDS are somewhat
beyond the scope of this book, but let us try to give you a quick intuition of
the ideas behind it. Imagine you were asked to draw a map showing the locations
of three towns—A, B, and C—and you were told that the distance from A to C is
200 miles, while the distances from A to B and B to C are 100 miles each. In
that case, you could conclude that towns A, B, and C must lie on a single
straight line, with B between A and C. The map would therefore be “one-dimensional.”
But if the A-B and B-C distances turned out to be 150 miles each (in general,
if the sum of the A-B and B-C distances is larger than the AC distance), then
you can conclude that A, B, and C cannot lie on a single (one-dimensional)
straight line, but rather must be arranged in a triangle in a (two-dimensional)
plane. Using considerations of this sort, MDS measures whether it is possible
to “embed” a given number of points into a space of a low number of dimensions
without seriously distorting (“straining”) their pairwise
distances.
If cochlear place and pulse rate
both affected the same single perceptual variable, that is, “pitch,” then it
ought to be possible to arrange all the perceptual distances between the
stimuli used in the experiment by Tong et al. (1983) along a single, one-dimensional
“perceptual pitch axis.” However, the results of the MDS analysis showed this
not to be possible. Only a two-dimensional perceptual space (shown on the right
in figure 8.7) can accommodate the
observed pairwise perceptual distances between the
stimuli used by Tong and colleagues. The conclusion from this experiment is
clear: When asked to rank stimuli from “high” to “low,” implantees
might report both changes in the place of stimulation to more basal locations
and increases in the pulse rate as producing a “higher” sound, but there seem
to be two different directions in perceptual space along which a stimulus can
“become higher.”
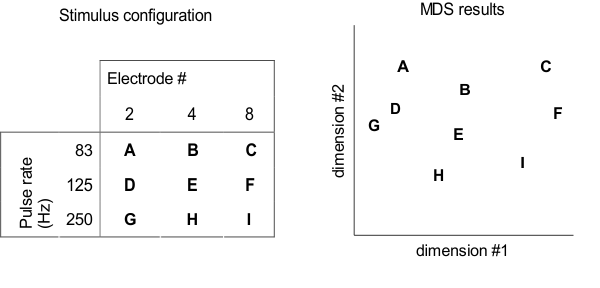
Figure 8.7
Perceptual
multidimensional scaling (MDS) experiment by Tong and colleagues (1983). Cochlear implant users were asked
to rank the dissimilarity of nine different stimuli (A–I), which differed in
pulse rates and cochlear locations, as shown in the table on the left. MSD
analysis results of the perceptual dissimilarity (distance) ratings, shown on
the right, indicate that pulse rate and cochlear place change the implantee’s sound percept along two independent dimensions.
A number of authors writing on
cochlear implant research have taken to calling the perceptual dimension
associated with the locus of stimulation “place pitch,” and that which is
associated with the rate of stimulation “periodicity pitch.” Describing two
demonstrably independent (orthogonal) perceptual dimensions as two different
“varieties” of pitch seems to us an unfortunate choice. If we present normal
listeners with a range of artificial vowels, keep the fundamental frequency
constant but shift some of the formant frequencies upward to generate something
resembling a /u/ to /i/ transition, and then pressed
our listeners to tell us which of the two vowels sounded “higher,” most would
reply that the /i/, with its larger acoustic energy
content at higher formant frequencies, sounds “higher” than the /u/. If we then
asked the same listeners to compare a /u/ with a fundamental frequency of 440
Hz with another /u/ with a fundamental frequency of 263 Hz, the same listeners
would call the first one higher. But only in the second case, the “periodicity
pitch” case where the fundamental frequency changes from the note C4
to A4 while the formant spectrum remains constant, are we dealing
with “pitch” in the sense of the perceptual quality that we use to appreciate musical
melody. The “place pitch” phenomenon, which accompanies spectral envelope
changes rather than changes in fundamental frequency, is probably better
thought of as an aspect of timbre, rather than a type of pitch.
The use of the term “place pitch”
has nevertheless become quite widespread, and as long as this term is used
consistently and its meaning is clear, such usage, although in our opinion not
ideal, is nevertheless defensible. Perhaps more worrying is the fact that one
can still find articles in the cochlear implant literature that simply equate
the term “pitch” with place of cochlear stimulation, without any reference to
temporal coding or further qualification. Given the crucial role temporal
discharge patterns play in generating musical pitch, that
is simply wrong.
With the concepts of “place pitch”
and “periodicity pitch” now clear and fresh in our minds, let us return to the
question of why pitch perception is generally poor in cochlear implant
recipients, and what might be done to improve it. An inability to appreciate
musical melody is one of the most common complaints of implantees,
although their ability to appreciate musical rhythms is very good. (On the
book’s website you can find examples of noise vocoded
pieces of music, which may give you an impression of what music might sound
like through a cochlear implant <flag>). Furthermore, speakers of tonal languages,
such as Mandarin, find it harder to obtain good speech recognition results with
cochlear implants (Ciocca et al., 2002). However,
melody appreciation through electrical hearing is bound to stay poor unless the
technology evolves to deliver more detailed temporal fine structure information
about a sound’s periodicity. Indeed, a number of experimental speech processor
strategies are being developed and tested, which aim to boost temporal
information by increasing the signals’ depth of modulation, as well as
synchronizing stimulus pulses relative to the sound’s fundamental frequency
across all electrode channels (Vandali et al., 2005).
These do seem to produce a statistically significant but nevertheless modest
improvement over conventional speech processing algorithms.
What exactly needs to be done to
achieve good periodicity pitch coding in cochlear implants remains somewhat
uncertain. As we mentioned earlier, electrical stimulation of the cochlea with
regular pulse trains of increasing frequency from 50 to 300 Hz produces a
sensation of increasing pitch (McKay, 2004; Shannon, 1983), but unfortunately, increasing
the pulse rates beyond 500 Hz usually does not increase the perceived pitch
further (Moore & Carlyon, 2005). In contrast, as we saw in chapter 3, the
normal (“periodicity”) pitch range of healthy adults extends up to about 4 kHz.
Why is the limit of periodicity pitch that can be easily achieved with direct
electrical stimulation through cochlear implants so much lower than that
obtained with acoustic click-trains in the normal ear?
One possibility that was
considered, but discounted on the basis of psychoacoustical
evidence, is that the basal, and hence normally high-frequency, sites stimulated
by cochlear implants may simply not be as sensitive to temporal patterning in
the pitch range as their low frequency, apical neighbors (Carlyon & Deeks, 2002). One likely alternative explanation is that
electrical stimulation may produce an excessive level of synchronization of
activity in the auditory nerve, which prevents the transmission of temporal
fine structure at high rates. Recall that, in the normal ear, each inner hair
cell connects to about ten auditory nerve fibers, and hair cells sit so closely
packed that several hundred auditory nerve fibers would all effectively serve
more or less the same “frequency channel.” This group of several hundred fibers
operates according to the “volley principle,” that is, while they phase lock to
the acoustic signal, an individual auditory nerve fiber need not respond to
every period of the sound. If it skips the odd period, the periods it misses will
very likely be marked by the firing of some other nerve fiber that forms part
of the assembly. Being able to skip periods is important, because physiological
limitations such as refractoriness mean that no nerve fiber can ever fire
faster than 1,000 Hz, and few are able to maintain firing rates greater than a
few hundred hertz for prolonged periods. Consequently, the temporal encoding of
the periodicity of sounds with fundamental frequencies greater than a few
hundred hertz relies on effective operation of the volley principle, so that
nerve fibers can “take it in turns” to mark the individual periods. In other
words, while we want the nerve fibers to lock to the periodicity of the sound,
we do not want them to be synchronized to each other.
It is possible that the physiology
of the synapses that connect the nerve fibers to the inner hair cells may favor
such an asynchronous activation. In contrast, electrical current pulses from
extracellular electrodes that, relative to the spatial scale
of individual nerve fibers, are both very large and far away, can only
lead to a highly synchronized activation of the nerve fibers, and would make it
impossible for the auditory nerve to rely on the volley principle for the
encoding of high pulse rates. Recordings from the auditory nerve of implanted
animals certainly indicate highly precise time locking to every pulse in a
pulse-train, as well as an inability to lock to rates higher than a few hundred
hertz (Javel et al., 1987), and recordings in the
central nervous system in response to electrical stimulation of the cochlea
also provide indirect evidence for a hypersynchronization
of auditory nerve fibers (Hartmann & Kral, 2004).
If this hypersynchronization
is indeed the key factor limiting pitch perception through cochlear implants,
then technical solutions to this problem may be a long way off. There have been
attempts to induce a degree of “stochastic resonance” to desynchronize the
activity of the auditory nerve fibers by introducing small amounts of noise or
jitter into the electrode signals, but these have not yet produced significant
improvements in pitch perception through cochlear implants (Chen, Ishihara,
& Zeng, 2005). Perhaps the contacts of electrodes
designed for insertion into the scala tympani are
simply too few, too large, and too far away from the spiral ganglion to allow
the activation of auditory nerve fibers in a manner that favors the
stimulus-locked yet desynchronized activity necessary to transmit a lot of
temporal fine structure information at high rates. In that case, “proper” pitch
perception through cochlear implants may require a radical redesign of the
implanted electrodes, so that many hundreds, rather than just a few dozen,
distinct electrical channels can be delivered in a manner that allows very
small groups of auditory nerve fibers to be targeted individually and activated
independently of their neighbors.
You may have got the impression
that using cochlear implants to restore hearing, or, in the case of the
congenitally deaf, to introduce it for the first time, involves starting with a
blank canvas in which the patient has no auditory sensation. This is not always
the case, however, as some residual hearing, particularly at low frequencies (1
kHz or less), may still be present. Given the importance of those low frequencies
in pitch perception, modified cochlear electrode arrays are now being used that
focus on stimulating the basal, dead high-frequency region of the cochlea while
leaving hearing in the intact low-frequency region to be boosted by
conventional hearing aids.
8.6 Spatial Hearing with Cochlear Implants
Until recently,
cochlear implantation was reserved solely for patients with severe hearing loss
in both ears, and these patients would typically receive an implant in one ear
only. The decision to implant only one ear was motivated partly from
considerations of added cost and surgical risk, but also from doubts about the
added benefit a second device might bring, and the consideration that, at a
time when implant technology was developing rapidly, it might be worth
“reserving” the second ear for later implantation with a more advanced device.
While unilateral implantation remains the norm at the time of this writing,
attitudes are changing rapidly in favor of bilateral implantation. Indeed, in
2008, the British National Institute for Health and Clinical Excellence (NICE)
changed its guidelines, and now recommends that profoundly deaf patients should
routinely be considered for bilateral implantation.
You may recall from chapter 5 that
binaural cues play a key role in our ability to localize sounds in space and to
pick out sounds of interest among other competing sounds. Any spatial hearing
that humans are capable of with just one ear stems from a combination of
head-shadow effects and their ability to exploit rather subtle changes at the
high end of the spectrum, where direction-dependent filtering by the external
ear may create spectral-shape localization cues. Contrast this with the
situation for most cochlear implant patients who will receive monaural
stimulation via a microphone located above and behind the ear, which, for the
reasons discussed earlier, conveys very limited spectral detail because
typically not much more than half a dozen or so effectively separated frequency
channels are available at any time. It is therefore unsurprising that, with
just a single implant, patients are effectively unable to localize sound
sources or to understand speech in noisy environments. Communicating in a busy
restaurant or bar is therefore particularly difficult for individuals with
unilateral cochlear implants.
Their quality of life can however
sometimes be improved considerably, by providing cochlear implants in both
ears, and patients with bilateral cochlear implants show substantially improved
sound localization compared to their performance with either implant alone (Litovsky et al., 2006; van Hoesel
& Tyler, 2003). Sensitivity to ILDs can be as
good as that seen in listeners with normal hearing, although ITD thresholds
tend to be much worse, most likely because of the lack of temporal fine
structure information provided by the implants (van Hoesel
& Tyler, 2003). Speech-in-noise perception can also improve following
bilateral cochlear implantation. In principle, such benefits may accrue solely from
“better ear” effects: The ear on the far side of a noise source will be in a
sound shadow produced by the head, which can improve the signal-to-noise ratio if
the sounds of interest originates from a different direction
than the distracting noise. A listener with two ears may be able to benefit
from the better ear effect simply by attending to the more favorably positioned
ear (hence the name), but a listener with only one functional ear may have to turn
her head in awkward and uncomfortable ways if the sound source directions of
the target and noise sources are unfavorable. Furthermore, Long and colleagues
(2006) showed that patients with bilateral cochlear implants can experience
binaural unmasking on the basis of envelope-based ITDs, suggesting that they
should be able to use their binaural hearing to improve speech perception in
noisy environments beyond what is achievable by the better ear effect alone.
8.6 Brain Plasticity and Cochlear Implantation
You may recall from
chapter 7 that the auditory system is highly susceptible to long-term changes
in input. A very important issue for the successful outcome of cochlear
implantation is therefore the age at which hearing is lost and the duration of
deafness prior to implantation. We know, for example, that early sensorineural hearing loss can cause neurons in the central
auditory system to degenerate and die (Shepherd & Hardie,
2000), and can also alter the synaptic and membrane properties of those neurons
that survive (Kotak, Breithaupt,
& Sanes, 2007). Neural pathways can also be
rewired, especially if hearing is lost on one side only (Hartley & King,
2010). Since bilateral cochlear implantees often
receive their implants at different times, their auditory systems will
potentially have to endure a period of complete deafness followed by deafness
in one ear.
A number of studies highlight the
importance of early implantation for maximizing the benefits of electrical
hearing. The latencies of cortical auditory evoked potentials reach normal
values only if children are implanted before a certain age (Sharma & Dorman,
2006), while studies in deaf cats fitted with a cochlear implant have also
shown that cortical response plasticity declines with age (Kral
& Tillein, 2006). Another complication is that
the absence of sound-evoked inputs, particularly during early development,
results in the auditory cortex being taken over by other sensory modalities (Doucet et al., 2006; Lee et al., 2007). Now cross-modal
reorganization can be very useful, making it possible, for example, for blind
patients to localize sounds more accurately (Röder et
al., 1999), or for deaf people to make better use of visual speech. On the
other hand, if auditory areas of the brain in the deaf are taken over by other
sensory inputs, the capacity of those areas to process restored auditory inputs
provided by cochlear implants may be limited.
We have discussed several examples
in this book where auditory and visual information is fused in ways that can
have profound effects on perception. A good example of this is the McGurk effect, in which viewing someone articulating one
speech sound while listening to another sound can change what we hear (see chapter
4 and accompanying video clip on the book’s web site). If congenitally deaf
children are fitted with cochlear implants within the first two and a half
years of life, they experience the McGurk effect.
However, after this age, auditory and visual speech cues can no longer be fused
(Schorr et al., 2005), further emphasizing the importance
of implantation within a sensitive period of development. Interestingly,
patients who received cochlear implants following postlingual
deafness—who presumably benefitted from multisensory experience early in
life—are better than listeners with normal hearing at fusing visual and
auditory signals, which improves their understaning
of speech in situations where both sets of cues are present (Rouger et al., 2007).
On the basis of the highly dynamic
way in which the brain processes auditory information, it seems certain that
the capacity of patients to interpret the distorted signals provided by
cochlear implants will be enhanced by experience and training strategies that
encourage their use in specific auditory tasks. Indeed, it is almost certainly
only because the brain possesses such remarkable adaptive capabilities that
cochlear implants work at all.