7 Development,
Learning, and Plasticity
- 7.1 When Does Hearing Start?
- 7.2 Hearing Capabilities Improve after Birth
- 7.3 The Importance of Early Experience: Speech and Music
- 7.4 Maturation of Auditory Circuits in the Brain
- 7.5 Plasticity in the Adult Brain
We have so far
considered the basis by which the auditory system can detect, localize, and
identify the myriad sounds that we might encounter. But how does our perception
of the acoustic environment arise during development? Are we born with these
abilities or do they emerge gradually during childhood? It turns out that much
of the development of the auditory system takes places before birth, enabling
many species, including humans, to respond to sound as soon as they are born.
Nonetheless, the different parts of the ear and the central auditory pathways
continue to mature for some time after that. This involves a lot of remodeling
in the brain, with many neurons failing to survive until adulthood and others
undergoing changes in the number and type of connections they form with other
neurons. Not surprisingly, these wiring modifications can result in
developmental changes in the auditory sensitivity of the neurons. As a
consequence, auditory perceptual abilities mature over different timescales, in
some cases not reaching the levels typically seen in adults until several years
after birth.
A very important factor in the
development of any sensory system is that the anatomical and functional
organization of the brain regions involved is shaped by experience during
so-called sensitive or critical periods of early postnatal life. This
plasticity helps to optimize those circuits to an individual’s sensory environment.
But this also means that abnormal experience—such as a loss of hearing in
childhood—can have a profound effect on the manner in which neurons respond to
different sounds, and therefore on how we perceive them.
Although sensitive periods of
development have been described for many species and for many aspects of
auditory function, including the emergence of linguistic and musical abilities,
we must remember that learning is a lifelong process. Indeed, extensive
plasticity is seen in the adult brain, too, which plays a vital function in
enabling humans and animals to interact effectively with their acoustic
environment and provides the basis on which learning can improve perceptual
abilities.
7.1 When Does Hearing Start?
The development of the
auditory system is a complex, multistage process that begins in early embryonic
life. The embryo comprises three layers, which interact to produce the various
tissues of the body. One of these layers, the ectoderm, gives rise to both
neural tissue and skin. The initial stage in this process involves the
formation of the otic placode,
a thickening of the ectoderm in the region of the developing hindbrain. As a
result of signals provided by the neural tube, from which the brain and spinal
cord are derived, and by the mesoderm, the otic placode is induced to invaginate
and fold up into a structure called the otocyst, from
which the cochlea and otic ganglion cells—the future
auditory nerve—are formed. Interestingly, the external ear and the middle ear
have different embryological origins from that of the inner ear. As a
consequence, congenital abnormalities can occur independently in each of these
structures.
The neurons that will become part
of the central auditory pathway are produced within the ventricular zone of the
embryo’s neural tube, from where they migrate to their final destination in the
brain. Studies in animals have shown that the first auditory neurons to be
generated give rise to the cochlear nucleus, superior olivary
complex, and medial geniculate nucleus, with the
production of neurons that form the inferior colliculus
and auditory cortex beginning slightly later. In humans, all the subcortical auditory structures can be recognized by the eighth
fetal week. The cortical plate, the first sign of the future cerebral cortex,
also emerges at this time, although the temporal lobe becomes apparent as a
distinct structure only in the twenty-seventh week of gestation (Moore & Linthicum,
2009).
To serve their purpose, the newly
generated neurons must make specific synaptic connections with other neurons.
Consequently, as they are migrating, the neurons start to send out axons that
are guided toward their targets by a variety of molecular guidance cues those
structures produce. These molecules are detected by receptors on the exploring
growth cones that form the tips of the growing axons, while other molecules
ensure that axons make contact with the appropriate region of the target
neurons. Robust synaptic connections can be established at an early stage—by
the fourteenth week of gestation in the case of the innervation
of hair cells by the spiral ganglion cells. On the other hand, another seven
weeks elapse before axons from the thalamus start to
make connections with the cortical plate.
At this stage of development, the
axons lack their insulating sheaths of myelin, which are required for the rapid
and reliable conduction of action potentials that is so important in the adult
auditory system. In humans, myelination of the
auditory nerve and the major brainstem pathways begins at the twenty-sixth week
of gestation, and it is at around this age that the first responses to sound
can be measured. One way of showing this is to measure event-related potentials
from the scalp of premature infants born soon after this age. But even within
the womb it is possible to demonstrate that the fetus can hear by measuring the
unborn baby’s movements or changes in heart rate that occur in response to vibroacoustic stimulation applied to the mother’s abdomen.
Such measurements have confirmed that hearing onset occurs at around the end of
the second trimester.
External sounds will, of course,
be muffled by the mother’s abdominal wall and masked by noises produced by her
internal organs. It is therefore perhaps not immediately clear what types of
sound would actually reach the fetus. Attempts to record responses from the
inner ear of fetal sheep, however, suggest that low-frequency speech could be
audible to human infants (Smith et al., 2003), and there is some evidence that,
toward the end of pregnancy, the human fetus not only responds to but can even
discriminate between different speech sounds (Shahidullah
& Hepper, 1994).
7.2 Hearing Capabilities Improve after Birth
Because of the
extensive prenatal development of the auditory system, human infants are born
with a quite sophisticated capacity to make sense of their auditory world. They
can readily distinguish between different phonemes, and are sensitive to the
pitch and rhythm of their mother’s voice. Within a few days of birth, babies
show a preference for their mother’s voice over that of another infant’s
mother, presumably as a result of their prenatal experience (DeCasper & Fifer, 1980). Perhaps more surprisingly,
various aspects of music perception can be demonstrated early in infancy. These
include an ability to distinguish different scales and chords and a preference
for consonant or pleasant-sounding intervals, such as the perfect fifth, over
dissonant intervals (Trehub, 2003), as well as
sensitivity to the beat of a rhythmic sound pattern (Winkler et al., 2009).
Whether these early perceptual
abilities are unique to human infants or specifically related to language and
music is still an open question. Mark Hauser at
It would be wrong to conclude from
this, however, that human infants can hear the world around them in the same
way that adults do. Almost all auditory perceptual abilities improve gradually
after birth, and the age at which adult performance is reached varies greatly
with the task. For example, sounds have to be played at a greater intensity to
evoke a response from an infant, and, particularly for low-frequency tones, it
can take as long as a decade until children possess the same low detection
thresholds seen in adults. The capacity to detect a change in the frequency of
two sequentially played tones also continues to improve over several years,
although frequency resolution—the detection of a tone of one frequency in the
presence of masking energy at other frequencies—seems to mature earlier.
Another aspect of hearing that
matures over a protracted period of postnatal development is sound
localization. While parents will readily attest to the fact that newborn
infants can turn toward their voices, the accuracy of these orienting responses
increases with age. Figure 7.1
shows that the minimum audible angle—the smallest
detectable change in sound source location—takes about 5 years to reach adult
values. As we saw in chapter 5, having two ears also helps in detecting
particular sounds against a noisy background. The measure of this ability, the
binaural masking level difference, takes at least 5 years and possibly much
longer to mature (Hall III, Buss, & Grose, 2007).
This is also the case for the precedence effect (Litovsky,
1997), indicating that the capacity to perceive sounds
in the reverberant environments we encounter in our everyday lives emerges over
a particularly long period.
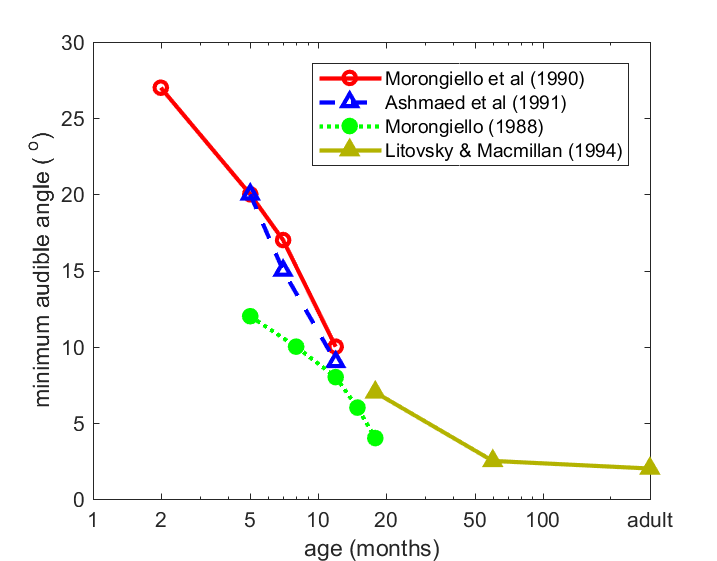
Figure 7.1
Minimum audible
angles, a measure the smallest change in the direction of a sound source that
can be reliably discriminated, decrease with age in humans.
From
Highly relevant to the perception
of speech and music is the development of auditory temporal processing.
Estimates of the minimum time period within which different acoustic events can
be distinguished have been obtained using a variety of methods. These include
the detection of amplitude and frequency modulation, gap detection, and nonsimultaneous masking paradigms. Although quite wide
variations have been found in the age at which adult values are reached with
the precise task and type of sound used, it is clear that temporal resolution
also takes a long time to reach maturity. For example, “backward masking,”
which measures the ability of listeners to detect a tone that is followed
immediately by a noise, has been reported to reach adult levels of performance
as late as 15 years of age.
To make sense of all this, we need
to take several factors into account. First, there is, of course, the
developmental status of the auditory system. Although the ear and auditory
pathways are sufficiently far advanced in their development to be able to
respond to sound well before birth in humans, important and extensive changes
continue to take place for several years into postnatal life. Second, nonsensory or cognitive factors will contribute to the
performance measured in infants. These factors include attention, motivation,
and memory, and they often present particular challenges when trying to assess
auditory function in the very young.
We can account for the maturation
of certain hearing abilities without having to worry about what might be
happening in the brain at the time. This is because changes in auditory
performance can be attributed to the postnatal development of the ear itself.
For instance, the elevated thresholds and relatively flat audiogram seen in
infancy are almost certainly due to the immature conductive properties of the external
ear and the middle ear found at that age. As these structures grow, the
resonant frequencies of the external ear decrease in value and the acoustic
power transfer of the middle ear improves. In both cases, it takes several
years for adult values to be reached, a timeframe consistent with age-related
improvements in hearing sensitivity.
Growth of the external ears and
the head also has considerable implications for sound localization. As we saw
in chapter 5, the auditory system determines the direction of a sound source
from a combination of monaural and binaural spatial cues. The values of those
cues will change as the external ears grow and the distance between them
increases (figure 7.2). As we shall see later,
when we look at the maturation of the neural circuits that process spatial
information, age-related differences in the cue values can account for the way
in which the spatial receptive fields of auditory neurons change during
development. In turn, it is likely that this will contribution to the gradual
emergence of a child’s localization abilities.
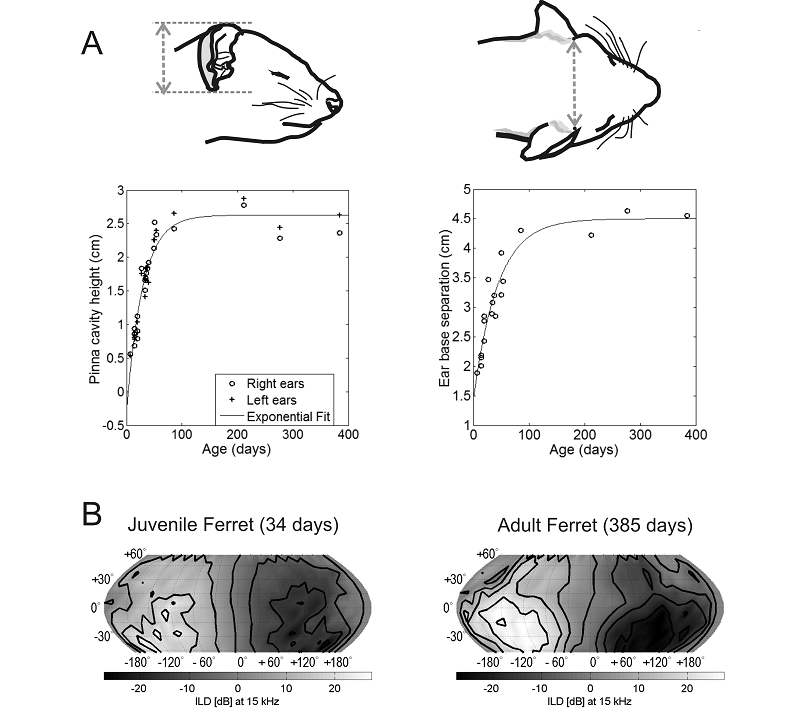
Figure 7.2
Growth of the head and
external ears changes the acoustic cue values corresponding to each direction
in space. (A) Age-related changes in the height of the external ear and in the
distance between the ears of the ferret, a species commonly used for studying auditory
development. These dimensions mature by around 4 months of age. (B) Variation
in interaural level differences (ILDs) for a 15-kHz
tone as a function of azimuth and elevation in a 34-day-old juvenile ferret
(left) and in an adult animal at 385 days of age (right). The range of ILDs is larger and their spatial pattern is somewhat
different in the older animal. Based on Schnupp,
Booth, and King (2003).
The range of audible frequencies
appears to change in early life as a result of developmental modifications in
the tonotopic organization of the cochlea. You should
now be very familiar with the notion that the hair cells near the base of the
cochlea are most sensitive to high-frequency sounds, whereas those located
nearer its apex are tuned to progressively lower frequencies. Although the
basal end of the cochlea matures first, studies in mammals and chicks have
shown that this region initially responds to lower sound frequencies than it
does in adults. This is followed by an increase in the sound frequencies to
which each region of the cochlea is most responsive, leading to an upward
expansion in the range of audible sound frequencies. Such changes have not been
described in humans, but it is possible that they take place before birth.
While the maturation of certain
aspects of auditory perception is constrained by the development of the ear,
significant changes also take place postnatally in
the central auditory system. An increase in myelination
of the auditory pathways results in a progressive reduction in the latency of
the evoked potentials measured at the scalp in response to sound stimulation.
At the level of the human brainstem, these changes are thought to be complete
within the first 2 years of life. However, Nina Kraus and colleagues have shown
that the brainstem responses evoked by speech sounds in 3- to 4-year-old
children are delayed and less synchronous than those recorded in older
children, whereas this difference across age is not observed with simpler
sounds (Johnson et al., 2008). But it is the neural circuits at higher levels
of the auditory system that mature most slowly, with sound-evoked cortical
potentials taking around 12 years to resemble those seen in adults (Wunderlich & Cone-Wesson, 2006).
7.3 The Importance of Early Experience: Speech and Music
Although it remains
difficult to determine how important the acoustic environment of the fetus is
for the prenatal development of hearing, there is no doubt that the postnatal
maturation of the central auditory pathways is heavily influenced by sensory
experience. Because this process is so protracted, there is ample opportunity
for the development of our perceptual faculties to be influenced by experience
of the sounds we encounter during infancy. As we shall see in the following
section, this also means that reduced auditory inputs, which can result, for
example, from early hearing loss, and even information provided by the other
senses can have a profound impact on the development of the central auditory
system.
The importance of experience in
shaping the maturing auditory system is illustrated very clearly by the
acquisition of language during early childhood. Infants are initially able to
distinguish speech sounds in any language. But as they learn from experience,
this languagewide capacity quickly narrows. Indeed,
during the first year of life, their ability to perceive phonetic contrasts in
their mother tongue improves, while they lose their sensitivity to certain
sound distinctions that occur only in foreign languages. You may remember from
chapter 4 that this is nicely illustrated by the classic example of adult
Japanese speakers, who struggle to distinguish the phonetic units “r” from “l,”
even though, at 7 months of age, Japanese infants are as adept at doing so as
native English speakers (figure 7.3).
In Japanese, these consonants fall within a single perceptual category, so
Japanese children “unlearn” the ability to distinguish them. This process of
becoming more sensitive to acoustic distinctions at phoneme boundaries of one’s
mother tongue, while becoming less sensitive to distinctions away from them,
has been found to begin as early as 6 months of age for vowels and by 10 months
for consonants (Kuhl & Rivera-Gaxiola,
2008). Perceptual narrowing based on a child’s experience during infancy is not
restricted to spoken language. Over the same time period, infants also become
more sensitive to the correspondence between speech sounds and the talker’s
face in their own language, and less so for non-native language (Pons et al.,
2009).
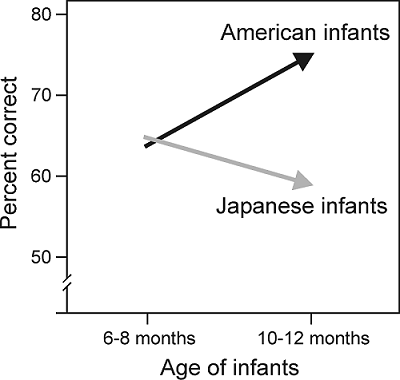
Figure 7.3
The
effects of age on speech perception performance in a cross-language study of
the perception of American English /r–l/ sounds by American and Japanese
infants.
From Kuhl et al. (2003).
These changes in speech perception
during the first year of life are driven, at least in part, by the statistical
distribution of speech sounds in the language to which the infant is exposed.
Thus, familiarizing infants with artificial speech sounds in which this
distribution has been manipulated experimentally alters their subsequent
ability to distinguish some of those sounds (Maye, Werker, & Gerken, 2002). But
social interactions also seem to play a role. Kuhl, Tsao, and Liu (2003) showed that 9-month-old American
infants readily learn phonemes and words in Mandarin Chinese, but only if they
were able to interact with a live Chinese speaker. By contrast, no learning
occurred if the same sounds were delivered by television or audiotape.
Not surprisingly, as a child’s
perceptual abilities become increasing focused on processing the language(s)
experienced during early life, the capacity to learn a new language declines.
In addition to the loss in the ability to distinguish phonemes in other
languages during the first year of life, other aspects of speech acquisition,
including the syntactic and semantic aspects of language, also appear to be
developmentally regulated (Ruben, 1997). The critical period of development
during which language can be acquired with little effort lasts for about 7
years. New language learning then becomes more difficult, despite the fact that
other cognitive abilities improve with age.
Sensitive periods have also been
characterized for other aspects of auditory development. Perhaps the most
relevant to language acquisition in humans is vocal learning in songbirds (figure
7.4). Young birds learn their songs by listening to adults
during an initial sensitive period of development, the duration of which varies
from one species to another and with acoustic experience and the level of
hormones such as testosterone. After this purely sensory phase of learning, the
birds start to make their own highly variable vocal attempts, producing what is
known as “subsong,” the equivalent of babbling in
babies. They then use auditory feedback during a sensorimotor
phase of learning to refine their vocalizations until a stable adult song is
crystallized (Brainard & Doupe,
2002).
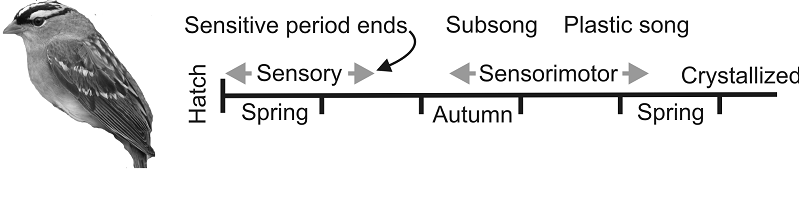
Figure 7.4
Birdsong
learning stages.
In seasonal species, such as the white-crowned sparrow, the sensory and sensorimotor phases of learning are separated in time. The
initial vocalizations (subsong) produced by young
birds are variable and generic across individuals. Subsong
gradually evolves into “plastic song,” which, although still highly variable,
begins to incorporate some recognizable elements of tutor songs. Plastic song
is progressively refined until the bird crystallizes its stable adult song. Other
songbirds show different time courses of learning.
From Brainard and Doupe (2002).
Thus, both human speech
development and birdsong learning rely on the individual being able to hear the
voices of others, as illustrated by Peter Marler’s
observation that, like humans, some songbirds possess regional dialects (Marler & Tamura, 1962). The importance of hearing the
tutor song during a sensitive period of development has been demonstrated by
raising songbirds with unrelated adults of the same species; as they mature,
these birds start to imitate the songs produced by the tutor birds. On the other
hand, birds raised in acoustic isolation—so that they are prevented from
hearing the song of conspecific adults—produce abnormal vocalizations. This is
also the case if songbirds are deafened before they have the opportunity to
practice their vocalizations, even if they have previously been exposed to
tutor songs; this highlights the importance of being able to hear their own
voices as they learn to sing. In a similar vein, profound hearing loss has a
detrimental effect on speech acquisition in children.
A related area where experience
plays a key role is in the development of music perception. We have already
pointed out that human infants are born with a remarkably advanced sensitivity
to different aspects of music. As with their universal capacity to distinguish
phonemes, infants initially respond in a similar way to the music of any
culture. Their perceptual abilities change with experience, however, and become
increasingly focused on the style of music to which they have been exposed. For
example, at 6 months of age, infants are sensitive to rhythmic variations in
the music of different cultures, whereas 12-month-olds show a culture-specific
bias (Hannon & Trehub, 2005). The perception of
rhythm in foreign music can nonetheless be improved at 12 months by brief
exposure to an unfamiliar style of music, whereas this is not the case in
adults.
Findings such as these again point
to the existence of a sensitive period of development during which perceptual
abilities can be refined by experience. As with the maturation of speech
perception, passive exposure to the sounds of a particular culture probably
leads to changes in neural sensitivity to the structure of music. But we also have
to consider the role played by musical training. In chapter 3, we introduced
the concept of absolute pitch—the ability to identify the pitch of a sound in
the absence of a reference pitch. It seems likely that some form of musical
training during childhood is a requirement for developing absolute pitch, and the
likelihood of having this ability increases if that training starts earlier.
This cannot, however, be the only explanation, as not all trained musicians
possess absolute pitch. In addition, genetic factors play a role in determining
whether or not absolute pitch can be acquired.
There is considerable interest in
being able to measure what actually goes on in the brain as auditory perceptual
abilities change during development and with experience. A number of noninvasive
brain imaging and electrophysiological recording methods are available to do
this in humans. Using these approaches, it has been shown that although
language functions are lateralized at birth, the regions of the cerebral cortex
involved are less specialized and the responses recorded from them are much
slower in infants than they are in adults (Friederici,
2006; Kuhl & Rivera-Gaxiola,
2008). Event-related potentials (ERPs) are particularly
suitable for studying time-locked responses to speech in young children. ERP
measurements suggest that by 7.5 months of age, the brain is more sensitive to
phonetic contrasts in the child’s native language than in a non-native language
(figure 7.5; Kuhl
& Rivera-Gaxiola, 2008). This is line with
behavioral studies of phonetic learning. Intriguingly, the differences seen at
this age in the ERP responses to native and non-native contrasts seem to
provide an indicator of the rate at which language is subsequently acquired.
Neural correlates of word learning can be observed toward the end of the first
year of life, whereas violations of syntactic word order result in ERP
differences at around 30 months after birth.
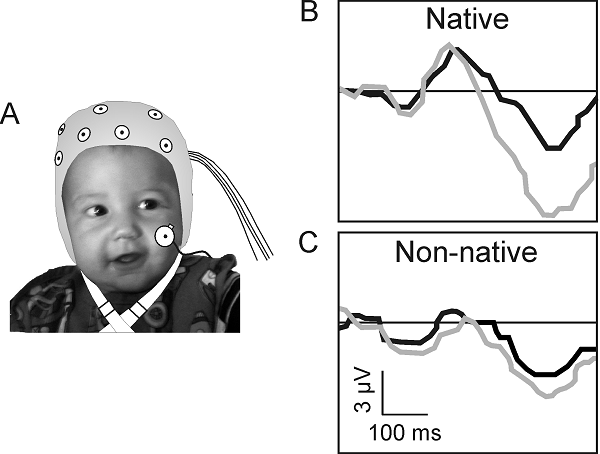
Figure 7.5
Neural correlates of
speech perception in infancy. (A) Human infant aged 7.5 months wearing an ERP electrocap. (B) ERP waveforms recorded at this age from one
sensor location in response to a native (English) and non-native (Mandarin
Chinese) phonetic contrast. The black waveforms show the response to a standard
stimulus, whereas the gray waveforms show the response to the deviant stimulus.
The difference in amplitude between the standard and deviant waveforms is
larger in the case of the native English contrast, implying better
discrimination than for the non-native speech sounds.
Adapted from Kuhl and Rivera-Gaxiola (2008).
Some remarkable examples of brain
plasticity have been described in trained musicians. Of course, speech and
music both involve production (or playing, in the case of a musical instrument)
as much as listening, so it is hardly surprising that motor as well as auditory
regions of the brain can be influenced by musical training and experience. Neuroimaging studies have shown that musical training can
produce structural and functional changes in the brain areas that are activated
during auditory processing or when playing an instrument, particularly if
training begins in early childhood. These changes most commonly take the form
of an enlargement of the brain areas in question and enhanced musically related
activity in them. One study observed structural brain plasticity in motor and
auditory cortical areas of 6-year-old children who received 15 months of
keyboard lessons, which was accompanied by improvements in musically relevant
skills (Hyde et al., 2009). Because no anatomical differences were found before
the lessons started between these children and an age-matched control group, it
appears that musical training can have a profound effect on the development of
these brain areas. In fact, plasticity is not restricted to the cerebral cortex,
as functional differences are also found in the auditory brainstem of trained
musicians (Kraus et al., 2009).
Imaging studies have also provided
some intriguing insights into the basis of musical disorders. We probably all
know someone who is tone deaf or unable to sing in tune. This condition may
arise as a result of a reduction in the size of the arcuate
fasciculus, a fiber tract that connects the temporal and frontal lobes of the
cerebral cortex (Loui, Alsop, & Schlaug, 2009). Consequently, tone deafness, which is found
in about 10% of the population, is likely to reflect reduced links between the
brain regions involved in the processing of sound, including speech and music,
and those responsible for vocal production.
7.4 Maturation of Auditory Circuits in the Brain
To track the changes
that take place in the human brain during development and learning, we have to
rely on noninvasive measures of brain anatomy and function. As in the mature
brain, however, these methods tell us little about what is happening at the
level of individual nerve cells and circuits. This requires a different
approach, involving the use of more invasive experimental techniques in
animals. In this section, we look at some of the cellular changes that take
place during development within the central auditory pathway, and examine how
they are affected by changes in sensory inputs.
The connections between the spiral
ganglion cells and their targets in the cochlea and the brainstem provide the
basis for the tonotopic representation of sound
frequency within the central auditory system. These connections therefore have
to be organized very precisely, and are thought to be guided into place at a
very early stage of development by chemical signals released by the target
structures (Fekete & Campero,
2007). The action potentials that are subsequently generated by the axons are
not responsible just for conveying signals from the cochlea to the brain. They
also influence the maturation of both the synaptic endings of the axons,
including the large endbulbs of Held, which, as we
saw in chapter 5, are important for transmitting temporal information with high
fidelity, and the neurons in the cochlear nucleus (Rubel
& Fritzsch, 2002). This is initially achieved
through action potentials that are generated spontaneously, in the absence of
sound, which are critical for the survival of the cochlear nucleus neurons
until the stage at which hearing begins.
The earliest sound-evoked responses
are immature in many ways. During the course of postnatal development,
improvements are seen in the thresholds of auditory neurons, in their capacity
to follow rapidly changing stimuli, and in phase locking, while maximum firing
rates increase and response latencies decrease. Some of these response
properties mature before others, and the age at which they do so varies at
different levels of the auditory pathway (Hartley & King, 2010). Because it
is the last structure to mature, a number of studies have focused on the
development of the auditory cortex. Changes occur in the frequency selectivity
of cortical neurons during infancy, a process that is greatly influenced by the
acoustic environment. For example, Zhang, Bao, and Merzenich (2001) showed that exposing young rats to
repeated tones of one frequency led to a distortion of the tonotopic
map, with a greater proportion of the auditory cortex now devoted to that
frequency than to other values. This does not necessarily mean that the animals
now hear better at these frequencies though; they actually end up being less
able to discriminate sound frequencies within the enlarged representation, but
better at doing so for those frequencies where the tonotopic
map is compressed (Han et al., 2007). This capacity for cortical reorganization
is restricted to a sensitive period of development, and different sensitive
periods have been identified for neuronal sensitivity to different sound
features, which coincide with the ages at which those response properties
mature (Insanally et al., 2009). Linking studies such
as these, in which animals are raised in highly artificial and structured
environments, to the development of auditory perception in children is
obviously not straightforward. It is clear, however, that the coding of
different sounds by cortical neurons is very dependent on experience during
infancy, and this is highly likely to influence the emergence of perceptual
skills.
While the aforementioned studies
emphasize the developmental plasticity of the cortex, subcortical
circuits can also undergo substantial refinements under the influence of
cochlear activity. These changes can have a considerable impact on the coding
properties—particularly those relating to sound source localization—of auditory
neurons. Neural sensitivity to ILDs and ITDs has been
observed at the youngest ages examined in the LSO (Sanes
& Rubel, 1988) and MSO (Seidl
& Grothe, 2005), respectively. The inhibitory
projection from the MNTB to the LSO, which gives rise to neural sensitivity to ILDs, undergoes an activity-dependent reorganization during
the normal course of development (Kandler, 2004).
Many of the initial connections die off and those that remain, rather
bizarrely, switch from being excitatory to inhibitory before undergoing further
structural remodeling. In chapter 5, we saw that precisely timed inhibitory
inputs to the gerbil MSO neurons can adjust their ITD sensitivity, so that the
steepest—and therefore most informative—regions of the tuning functions lie
across the range of values that can occur naturally given the size of the head.
Seidl and Grothe (2005)
showed that in very young gerbils these glycinergic
synapses are initially distributed uniformly along each of the two dendrites of
the MSO neurons. A little later in development, they disappear, leaving
inhibitory inputs only on or close to the soma of the neurons, which is the
pattern seen in adult gerbils (figure 7.6).
This anatomical rearrangement alters the ITD sensitivity of the neurons, but
occurs only if the animals receive appropriate auditory experience. If they are
denied access to binaural localization cues, the infant distribution persists
and the ITD functions fail to mature properly.
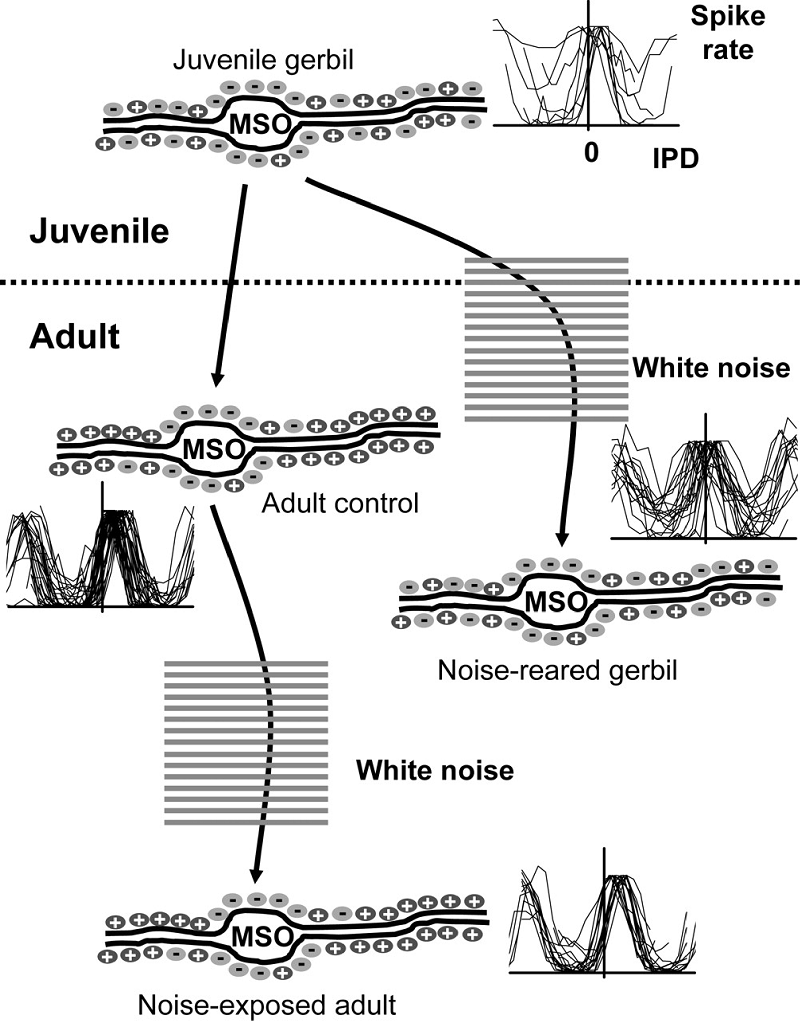
Figure 7.6
Maturation of
brainstem circuits for processing interaural time differences. In juvenile
gerbils, at around the time of hearing onset, excitatory and inhibitory inputs
are distributed on the dendrites and somata of
neurons in the medial superior olive (MSO). At this age, neurons prefer
interaural phase differences (IPD) around 0. In adult gerbils, glycinergic inhibition is restricted to the cell somata and is absent from the dendrites and IPD response
curves are shifted away from 0, so that the maximal slope lies within the
physiological range. This developmental refinement depends on acoustic
experience, as demonstrated by the effects of raising gerbils in omnidirectional white noise, which preserves the juvenile
state. Exposing adults to noise has no effect on either the distribution of glycinergic synapses on the MSO neurons or their IPD
functions.
Used with permission from Seidl and Grothe (2005).
Changes in binaural cue
sensitivity would be expected to shape the development of the spatial receptive
fields of auditory neurons and the localization behaviors to which they
contribute. Recordings from the superior colliculus (Campbell
et al., 2008) and auditory cortex (Mrsic-Flogel et
al., 2003) have shown that the spatial tuning of the neurons is indeed much
broader in young ferrets than it is in adult animals. But it turns out that
this is due primarily to the changes in the localization cue values that take
place as the head and ears grow. Thus, presenting infant animals with stimuli
through virtual adult ears led to an immediate sharpening in the spatial
receptive fields. This demonstrates that both peripheral and central auditory
factors have to be taken into account when assessing how adult processing
abilities are reached.
Nevertheless, it is essential that
the neural circuits involved in sound localization are shaped by experience. As
we have seen, the values of the auditory localization cues depend on the size
and shape of the head and external ears, and consequently will vary from one individual
to another. Each of us therefore has to learn to localize with our own ears.
That this is indeed the case has been illustrated by showing that humans
localize virtual space stimuli more accurately when the stimuli are generated
from acoustical measurements made from their own ears than from the ears of
other individuals (Wenzel et al., 1993).
Plasticity of auditory spatial
processing has been demonstrated by manipulating the sensory cues available.
For example, inducing a reversible conductive hearing loss by plugging one ear
will alter the auditory cue values corresponding to different directions in
space. Consequently, both sound localization accuracy and the spatial tuning of
auditory neurons will be disrupted. However, if barn owls (Knudsen, Esterly, & Knudsen, 1984) or ferrets (King, Parsons,
& Moore, 2000) are raised with a plug inserted in one ear, they learn to
localize sounds accurately (figure 7.7A).
Corresponding changes are seen in the optic tectum
(Knudsen, 1985) and superior colliculus (King et al.,
2000), where, despite the abnormal cues, a map of auditory space emerges in
register with the visual map (figure 7.7).
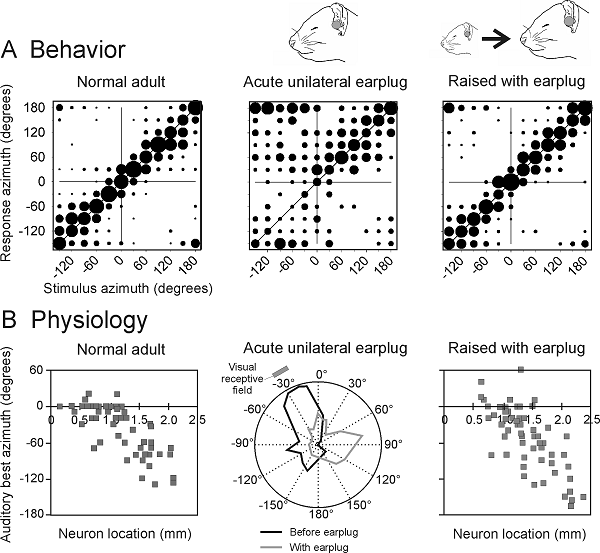
Figure 7.7
Auditory experience
shapes the maturation of sound localization behavior and the map of auditory
space in the superior colliculus. (A)
Stimulus-response plots showing the combined data of three normally reared
ferrets (normal adult ferrets), another three animals just after inserting an
earplug into the left ear (adult left earplug), and three ferrets that had been
raised and tested with the left ear occluded with a plug that produced 30- to 40-dB
attenuation (reared with left earplug). These plots illustrate the distribution
of approach-to-target responses (ordinate) as a function of stimulus location
(abscissa). The stimuli were bursts of broadband noise. The size of the dots
indicates, for a given speaker angle, the proportion of responses made to
different response locations. Occluding one ear disrupts sound localization
accuracy, but adaptive changes take place during development that enable the
juvenile plugged ferrets to localize sound almost as accurately as the
controls. (B) The map of auditory space in the ferret SC, illustrated by
plotting the best azimuth of neurons versus their location within the nucleus.
Occluding one ear disrupts this spatial tuning, but, as with the behavioral
data, near-normal spatial tuning is present in ferrets that were raised with
one ear occluded.
At the end of chapter 5, we
discussed the influence that vision can have over judgments of sound source
location in humans. A similar effect is also seen during development if visual
and auditory cues provide spatially conflicting information. This has been
demonstrated by providing barn owls with spectacles containing prisms that
shift the visual world representation relative to the head. A compensatory
shift in the accuracy of sound-evoked orienting responses and in the auditory
spatial receptive fields of neurons in the optic tectum
occurs in response to the altered visual inputs, which is brought about by a
rewiring of connections in the midbrain (figure 7.8; Knudsen,
1999). This experiment was possible because barn owls have a very limited
capacity to move their eyes. In mammals, compensatory eye movements would
likely confound the results of using prisms. Nevertheless, other approaches
suggest that vision also plays a guiding role in aligning the different sensory
representations in the mammalian superior colliculus
(King et al., 1988). Studies in barn owls have shown that experience-driven
plasticity is most pronounced during development, although the sensitive period
for visual refinement of both the auditory space map and auditory localization
behavior can be extended under certain conditions (Brainard
& Knudsen, 1998).
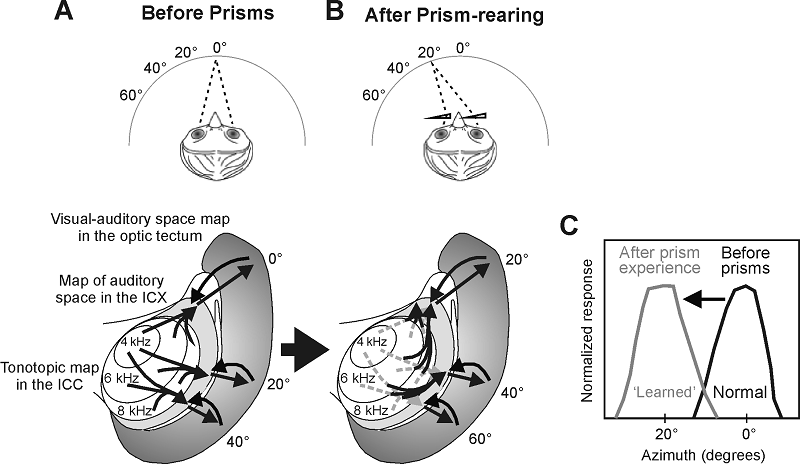
Figure 7.8
Visual experience
shapes the map of auditory space in the midbrain of the barn owl. (A) The owl's
inferior colliculus (ICX) contains a map of auditory
space, which is derived from topographic projections that combine spatial
information across different frequency channels in the central nucleus of the
inferior colliculus (ICC). The ICX map of auditory
space is then conveyed to the optic tectum, where it
is superimposed on a map of visual space. (B) From around 40 days after
hatching, the auditory space maps in both the optic tectum
and the ICX are refined by visual experience. This has been demonstrated by
chronically shifting the visual field in young owls by mounting prisms in front
of their eyes. The same visual stimulus now activates a different set of neurons
in the optic tectum. The auditory space maps in the
ICX and tectum gradually shift by an equivalent
amount in prism-reared owls, thereby reestablishing the alignment with the
optically displaced visual map in the tectum. This
involves growth of novel projections from the ICC to the ICX (unbroken lines);
the original connections remain in place but are suppressed (broken lines).
Finally, we need to consider how
complex vocalizations are learned. This has so far been quite difficult to
study in nonhuman species, but studies of birdsong learning have provided some
intriguing insights into the neural processing of complex signals that evolve
over time, and there is every reason to suppose that similar principles will
apply to the development of sensitivity to species-specific vocalizations in
mammals. In section 7.3, we described how vocal learning in songbirds is guided
by performance feedback during a sensitive period of development. Several
forebrain areas are thought to be involved in the recognition of conspecific
song. In juvenile zebra finches, neurons in field L, the avian equivalent of
the primary auditory cortex, are less acoustically responsive and less
selective for natural calls over statistically equivalent synthetic sounds than
they are in adult birds (Amin, Doupe,
& Theunissen, 2007). Neuronal selectivity for
conspecific songs emerges at the same age at which the birds express a
behavioral preference for individual songs (Clayton, 1988), implicating the
development of these response properties in the maturation of song recognition.
The auditory forebrain areas project to a cluster of structures, collectively
known as the “song system,” which have been shown to be involved in vocal
learning. Recording studies have shown that certain neurons of the song system
prefer the bird’s own song or the tutor’s song over other complex sounds,
including songs from other species (Margoliash,
1983), and that these preferences emerge following exposure to the animal’s own
vocal attempts (Solis & Doupe, 1999).
7.5 Plasticity in the Adult Brain
We have seen that many
different aspects of auditory processing and perception are shaped by
experience during sensitive periods of development. While the length of those
periods can vary with sound property, brain level, and species and may be
extended by hormonal or other factors, it is generally accepted that the
potential for plasticity declines with age. That would seem to make sense,
since more stability may be desirable and even necessary in the adult brain to
achieve the efficiency and reliability of a mature nervous system. But it turns
out that the fully mature auditory system shows considerable adaptive
plasticity that can be demonstrated over multiple timescales.
Numerous examples have been
described where the history of stimulation can determine the responsiveness or
even the tuning properties of auditory neurons. For example, if the same
stimulus, say a tone of a particular frequency, is presented repeatedly, neurons
normally show a decrease in response strength. However, the response can be
restored if a different frequency is presented occasionally (Ulanovsky, Las, & Nelken,
2004). This phenomenon is known as stimulus-specific adaptation, and
facilitates the detection of rare events and sudden changes in the acoustic
environment (see chapter 6). On the other hand, the sensitivity of the neurons
can change so that the most frequently occurring stimuli are represented more
precisely. A nice example of this “adaptive coding” was described by Dean and
colleagues (2005), who showed that the relationship between the firing rate of
inferior colliculus neurons and sound level can
change to improve the coding of those levels that occur with the highest
probability. One important consequence of this is that a greater range of sound
levels can be encoded, even though individual neurons have a relatively limited
dynamic range.
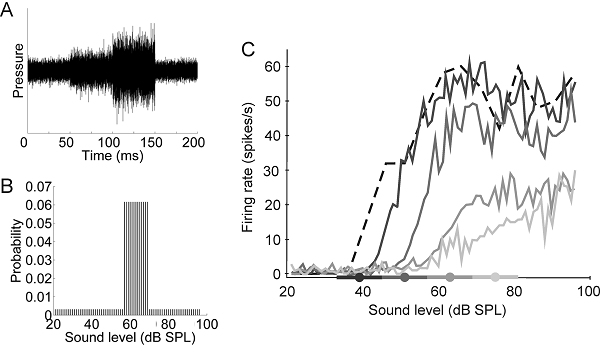
Figure 7.9
Adjustments in responses
of inferior collicular neurons to the mean ongoing
sound level. Broadband stimuli on which the sound level was
varied over a wide range, but with a high-probability region centered on
different values. (A) Distribution of sound levels in the stimulus with
a high-probability region centered on 63 dB SPL. (B) Rate-level functions for
one neuron for four different sound level distributions, as indicated by the
filled circles and thick lines on the x axis. Note that the functions shift as
the range of levels over which the high-probability region is presented
changes.
From Dean, Harper,
and McAlpine (2005).
These changes occur in passive
hearing conditions and are therefore caused solely by adjustments in the
statistics of the stimulus input. If a particular tone frequency is given
behavioral significance by following it with an aversive stimulus, such as a
mild electric shock, the responses of cortical neurons to that frequency can be
enhanced (Weinberger, 2004). Responses to identical stimuli can even change
over the course of a few minutes in different ways in a task-dependent fashion
(Fritz, Elhilali, & Shamma,
2005), implying that this plasticity may reflect differences in the meaning of
the sound according to the context in which it is presented.
Over a longer time course, the tonotopic organization of the primary auditory cortex of
adult animals can change following peripheral injury. If the hair cells in a
particular region of the cochlea are damaged as a result of exposure to a
high-intensity sound or some other form of acoustic trauma, the area of the
auditory cortex in which the damaged part of the cochlea would normally be
represented becomes occupied by an expanded representation of neighboring sound
frequencies (Robertson & Irvine, 1989). A similar reorganization of the
cortex has been found to accompany improvements in behavioral performance that
occur as a result of perceptual learning. Recanzone,
Schreiner, and Merzenich (1993) trained monkeys on a
frequency discrimination task and reported that the area of cortex representing
the tones used for training increased in parallel with improvements in
discrimination performance. Since then, a number of other changes in cortical
response properties have been reported as animals learn to respond to
particular sounds. This does not necessarily involve a change in the firing
rates or tuning properties of the neurons, as temporal firing patterns can be
altered as well (Bao et al., 2004; Schnupp et al., 2006).
There is a key difference between
the cortical changes observed following training in adulthood and those
resulting from passive exposure to particular sounds during sensitive periods
of development, in that the sounds used for training need to be behaviorally
relevant to the animals. This was demonstrated by Polley,
Steinberg, and Merzenich (2006), who trained rats
with the same set of sounds on either a frequency or a level recognition task.
An enlarged representation of the target frequencies was found in the cortex of
animals that learned the frequency recognition task, whereas the representation
of sound level in these animals was unaltered. By contrast, training to respond
to a particular sound level increased the proportion of neurons tuned to that
level without affecting their tonotopic organization.
These findings suggest that attention or other cognitive factors may dictate
how auditory cortical coding changes according to the behavioral significance
of the stimuli, which is thought to be signaled by the release in the auditory
cortex of neuromodulators such as acetylcholine.
Indeed, simply coupling the release of acetylcholine with sound stimulus in
untrained animals is sufficient to induce a massive reorganization of the adult
auditory cortex (Kilgard, 2003).
Training can also dramatically
improve the auditory perceptual skills of humans, and the extent to which
learning generalizes to other stimuli or tasks can provide useful insights into
the underlying neural substrates (Wright & Zhang, 2009). As in the animal
studies, perceptual learning in humans can be accompanied by enhanced responses
in the auditory cortex (Alain et al., 2007; van Wassenhove
& Nagarajan, 2007). It is not, however,
necessarily the case that perceptual learning directly reflects changes in
brain areas that deal with the representation of sound attribute in question.
Thus, a substantial part of the improvement in pitch discrimination during
perceptual learning tasks is nonspecific—for example, subjects playing a
computer game while hearing pure tones (but not explicitly attending to these
sounds) improve in pitch discrimination. The same was true even for subjects
who played a computer game without hearing any pure tones (Amitay,
Irwin, & Moore, 2006)! Such improvement must be due to general factors
governing task performance rather than to specific changes in the properties of
neurons in the auditory system.
In addition to improving the
performance of subjects with normal hearing, training can promote the capacity
of the adapt brain to adjust to altered inputs. This has been most clearly
demonstrated in the context of sound localization. In the previous section, we
saw that the neural circuits responsible for spatial hearing are shaped by
experience during the phase of development when the localization cues are
changing in value as a result of head growth. Perhaps surprisingly, the mature
brain can also relearn to localize sound in the presence of substantially
altered auditory spatial cues. Hofman et al. (1998)
showed that adult humans can learn to use altered spectral localization cues.
To do this, they inserted a mold into each external ear, effectively changing
its shape and therefore the spectral-shape cues corresponding to different
sound directions. This led to an immediate disruption in vertical localization,
with performance gradually recovering over the next few weeks (figure
7.10).
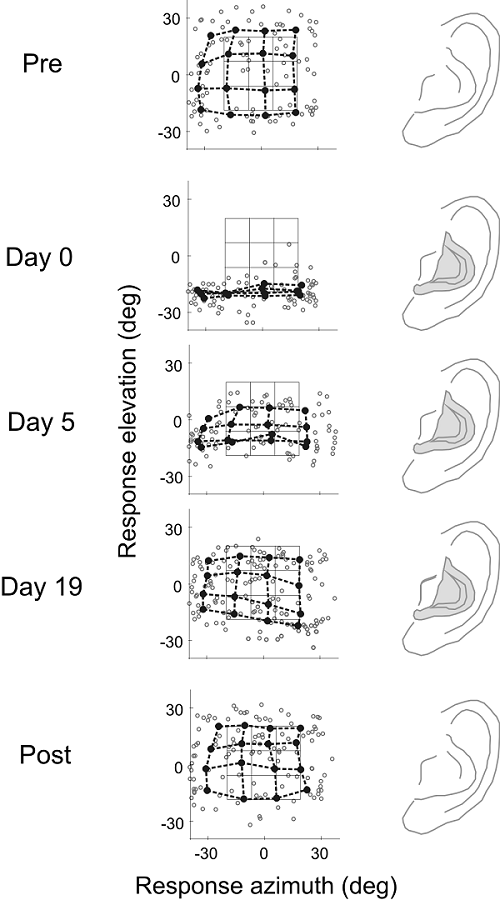
Figure 7.10
Learning
to localize sounds with new ears in adult humans. In this study, the accuracy of
sound localization was assessed by measuring eye movements made by human
listeners toward the location of broadband sound sources. The stimuli were
presented at random locations encompassed by the black grid in each of the
middle panels. The end points of all the saccadic eye movements made by one
subject are indicated by the red dots. The blue dots and connecting lines
represent the average saccade vectors for targets located within neighboring
sectors of the stimulus grid. The overlap between the response and target
matrices under normal listening conditions (precontrol)
shows that saccadic eye movements are quite accurate in azimuth and elevation.
Molds were then fitted to each external ear, which altered the spatial pattern
of spectral cues. Measurements made immediately following application of the molds
(day 0) showed that elevation judgments were severely disrupted, whereas
azimuth localization within this limited region of space were unaffected. The
molds were left in place for several weeks, and, during this period,
localization performance gradually improved before stabilizing at a level close
to that observed before the molds were applied. No aftereffect was observed
after the molds were removed (postcontrol), as the
subjects were able to localize sounds as accurately as they did in the precontrol condition.
From Hofman, Van Riswick, and Van Opstal (1998).
Because it relies much more on
binaural cues, localization in the horizontal plane becomes inaccurate if an
earplug is inserted in one ear. Once again, however, the mature auditory system
can learn to accommodate the altered cues. This has been shown in both humans (Kumpik, Kacelnik, & King,
2010) and ferrets (Kacelnik et al., 2006), and seems
to involve a reweighting away from the abnormal binaural cues so that greater
use is made of spectral-shape information. This rapid recovery of sound
localization accuracy occurs only if appropriate behavioral training is
provided (figure 7.11). It is also critically
dependent on the descending pathways from the auditory cortex to the midbrain (Bajo et al., 2010), which can modulate the responses of the
neurons found there in a variety of ways (Suga & Ma,
2003). This highlights a very important, and often ignored, aspect of auditory
processing, namely, that information passes down as well as up the pathway. As
a consequence of this, plasticity in subcortical as
well as cortical circuits is likely to be involved in the way humans and other
species interact with their acoustic environments.
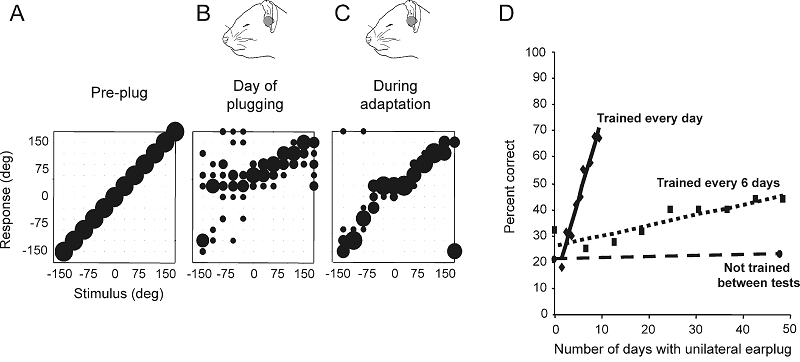
Figure 7.11
Plasticity
of spatial hearing in adult ferrets. (A–C) Stimulus-response plots showing the
distribution of responses (ordinate) made by a ferret as a function of stimulus
location in the horizontal plane (abscissa). The size of the dots indicates,
for a given speaker angle, the proportion of responses made to different
locations. Correct responses are those that fall on the diagonal line, whereas
all other responses represent errors of different magnitude. Prior to occlusion
of the left ear, the animal achieved 100% correct scores at all stimulus
directions (A), but performed poorly, particularly on the side of the earplug,
when the left ear was occluded (B). Further testing with the earplug still in
place, however, led to a recovery in localization accuracy (C). (D) Mean change
in performance (averaged across all speaker locations) over time in three
groups of ferrets with unilateral earplugs. No change was found in trained
ferrets (n = 3) that received an
earplug for 6 weeks, but were tested only at the start and end of this period (circles
and dashed regression line). Two other groups of animals received an equivalent
amount of training while the left ear was occluded. Although the earplug was in
place for less time, a much faster rate of improvement was observed in the
animals that received daily training (n
= 3; diamonds and solid regression line) compared to those that were tested
every 6 days (n = 6; squares and
dotted regression line).
From Kacelnik
et al. (2006)
We have seen in this chapter that
the auditory system possesses a truly remarkable and often underestimated
capacity to adapt to the sensory world. This is particularly the case in the
developing brain, when newly formed neural circuits are refined by experience
during specific and often quite narrow time windows. But the capacity to learn
and adapt to the constantly changing demands of the environment is a lifelong
process, which requires that processing in certain neural circuits can be
modified in response to both short-term and long-term changes in peripheral
inputs. The value of this plasticity is clear: Without it, it would not be
possible to customize the brain to the acoustical cues that underlie our
capacity to localize sound or for the processing of native language. But the
plasticity of the central auditory system comes at a potential cost, as this
means that a loss of hearing, particularly during development, can induce a
rewiring of connections and alterations in the activity of neurons, which could
give rise to conditions such as tinnitus, in which phantom sounds are
experienced in the absence of acoustic stimulation (Eggermont,
2008). At the same time, experience-dependent learning enhances the capacity of
the auditory system to accommodate the changes in input associated with hearing
loss and its restoration, a topic that we shall return to in the next chapter.